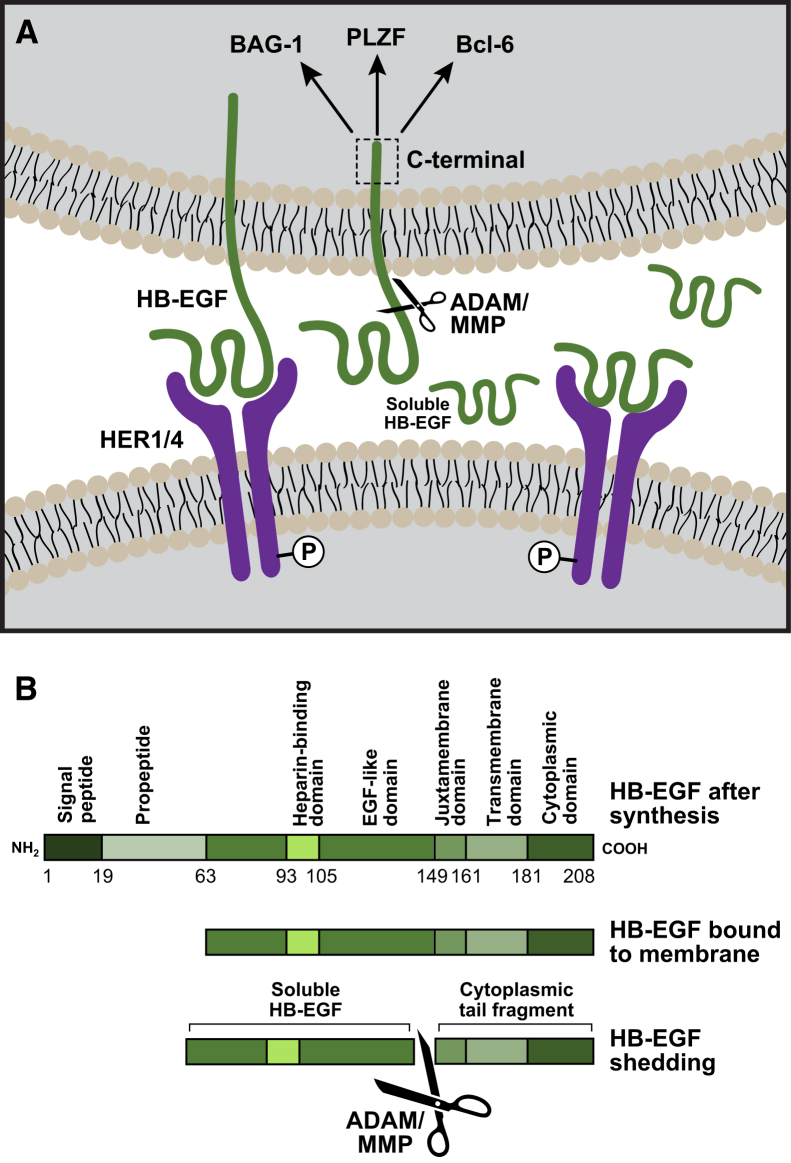
Figure 1.
Ectodomain shedding and processing of heparin-binding epidermal growth factor–like growth factor (HB-EGF). A: Illustration denotes two cells participating in juxtacrine signaling: top cell expresses membrane-bound pro-HB-EGF, and bottom cell expresses the receptor(s) for HB-EGF. Ectodomain shedding by matrix metalloproteinase (MMP) or a disintegrin and metalloproteinase (ADAM) generates soluble HB-EGF that can participate in autocrine or paracrine signaling. The cytoplasmic tail of HB-EGF (pro-HB-EGF cytoplasmic tail) can translocate to the nucleus (in the top cell) and interact directly or indirectly with proteins, such as Bcl-2–associated athanogene 1 (BAG-1), promyelocytic leukemia zinc finger (PLZF), and Bcl-6, to promote cellular proliferation. B: Molecular processing of pro-HB-EGF to membrane-bound HB-EGF and enzymatic cleavage to soluble HB-EGF. Initially after protein synthesis, pro-HB-EGF contains a signal peptide and a propeptide. Membrane-bound HB-EGF contains an amino terminal heparin-binding domain, an EGF-like domain, and a juxtamembrane domain on the extracellular region, whereas the transmembrane domain spans the membrane and the cytoplasmic C-terminal domain is inside the cell. Enzymes cleave HB-EGF between the EGF-like domain and the juxtamembrane region to form soluble HB-EGF. HER, human epidermal growth factor receptor; P, tyrosine phosphorylation of the receptor upon ligand binding.