Figure 5.
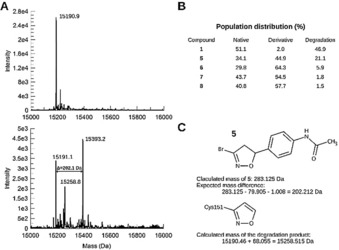
The isoxazoline covalently modifies a single cysteine in the BTB domain of Keap1. A) Deconvoluted intact protein mass spectrum of the native protein (top) and after treatment with 5 (bottom). The masses determined by MS are in good agreement with the theoretical mass for the native protein (15 190.46 Da) and with that of the protein after covalent modification of a cysteine residue of the protein by compounds. B) Population distribution between native unmodified protein, protein modified by the intact compound, and modified protein after elimination of the aryl substituent at position‐5 of the isoxazoline moiety. C) The theoretical and calculated masses are given for the example of the reaction of BTB with 5 and for the degradation product common to all compounds (Figure 5 B).
