Abstract
Clinically, low and moderate alcohol intake improves human health with protection against metabolic syndromes, including type 2 diabetes; however, mechanisms that are associated with these effects remain to be elucidated. The aims of this study were to investigate the effects of moderate alcohol intake on thermogenic brown/beige adipocyte formation and glucose and lipid homeostasis, as well as the involvement of retinoic acid (RA) signaling in the entire process. C57BL6 male mice were supplemented with 8% (w/v) alcohol in water for 1 or 4 mo. Alcohol intake prevented body weight gain, induced the formation of uncoupling protein 1–positive beige adipocytes in white adipose tissue, and increased thermogenesis in mice, which is associated with decreased serum glucose and triacylglycerol levels. Mechanistically, alcohol intake increased RA levels in serum and adipose tissue, which was associated with increased expression of aldehyde dehydrogenase family 1 subfamily A1 (Aldh1a1). When RA receptor-α signaling was conditionally blocked in platelet-derived growth factor receptor-α-positive adipose progenitors, the effects of alcohol on beige adipogenesis were largely abolished. Finally, moderate alcohol prevented high-fat diet-induced obesity and metabolic dysfunction. In conclusion, moderate alcohol intake induces thermogenic brown/beige adipocyte formation and promotes glucose and lipid oxidation via elevation of RA signaling.—Wang, B., Wang, Z., de Avila, J. M., Zhu, M.-J., Zhang, F., Gomez, N. A., Zhao, L., Tian, Q., Zhao, J., Maricelli, J., Zhang, H., Rodgers, B. D., Du, M. Moderate alcohol intake induces thermogenic brown/beige adipocyte formation via elevating retinoic acid signaling.
Keywords: beige adipocyte, glucose, fatty acids, thermogenesis, obesity
Obesity is a worsening problem worldwide and is closely linked to type 2 diabetes, cardiovascular diseases, and a number of cancers. In the United States, more than one third of the population is obese with another one third classified as overweight. Taken together, the occurrence of these conditions produces huge medical costs and overall loss of human capital (1); therefore, it has become critically important to control the obesity epidemic. Adipose tissues can be separated into 3 major types: white adipose tissue (WAT; white fat), brown adipose tissue (BAT; brown fat), and brown-like adipose tissue (beige fat) (2). The function of white fat is to store extra energy in the form of triacylglycerols, which are closely associated with obesity. In contrast, brown fat is mainly found in the interscapular region and dissipates energy as heat (3). This is achieved via expression of uncoupling protein 1 (UCP1). This protein resides in the inner mitochondrial membrane and diverts the energy potential of the proton ion gradient into heat production rather than ATP synthesis (4); however, the amount of brown fat that is typically found in adults is quite low, which casts doubt on its effect on the prevalence of obesity (5). Similar to brown adipocytes, beige adipocytes express UCP1 and are induced by cold environments and β-adrenergic signaling (6). Thus, browning, or the stimulation of beige adipocyte development, becomes a feasible method for the treatment of obesity independent of exercising (7).
Alcohol is a common beverage that accounts for as much as 8% of total average caloric intake (8). Because alcohol contains a large amount of energy and stimulates the appetite by increasing meal duration and sizes, a positive relationship between food intake and alcohol intake exists (9); however, the increase in food intake as a result of alcohol consumption does not translate into risk of obesity. In an epidemiologic study that examined 7230 U.S. adults, alcohol intake did not increase obesity risk (10). Daily red wine consumption does not promote obesity (11), whereas those who consume low and moderate amounts of alcohol decreased rates of obesity (12). French people commonly consume high-energy diets coupled with a frequent consumption of wine, yielding no increase in the incidence of cardiovascular disease and obesity—termed the French paradox because of the lack of understanding of the mechanisms that underlie this observation (13). Clinical evidence suggests that low and moderate alcohol intake has beneficial effects on human health, such as protection against nonalcoholic fatty liver disease (14, 15) and decreased risk of type 2 diabetes (16). In addition, short-term oral exposure to white wine lowers serum free fatty acids (17) and improves insulin sensitivity (18). Despite the accumulating evidence, the underlying mechanisms that are responsible for the beneficial effects of moderate alcohol intake on obesity and metabolic health remain poorly understood.
Alcohol and retinol (ROL) share common metabolic enzymes that are involved in their respective conversions into acetic acid and retinoic acid (RA) (19). In mice, supplementation of ethanol increases physiologic all-trans-RA content in adult tissues (20) and reduces ROL storage in the liver (21). This is postulated to be a result of the stimulation of alcohol metabolism enzyme activity by alcohol intake, which promotes conversion of ROL to RA. Retinoids are involved in preadipocyte commitment (22) and adipocyte maturation. Recently, we found that RA prevents white adipogenesis by inhibiting zinc-finger protein 423 (Zfp423) (23). It has also been shown that RA induces lipid oxidation by promoting UCP1 expression and brown adipogenesis (24, 25). The aims of this study were to investigate the effects of moderate alcohol intake on thermogenic brown/beige adipocyte formation and glucose and lipid homeostasis, as well as to understand the underlying mechanism in mouse models.
MATERIALS AND METHODS
Mice
Animal studies were conducted in Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities and according to protocols that were approved by the Institutional Animal Care and Use Committee. C57BL/6 mice and platelet-derived growth factor receptor (PDGFR)α-cre/ERT mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). ROSA26-RARαDN mice were kindly provided by Dr. Cathy Mendelsohn (Columbia University, New York, NY, USA) (26).
Animals were housed in a temperature- and humidity-controlled room on a 12-h light/dark cycle. A commercial grain alcohol (Everclear grain alcohol, 151 proof, 4.16 kcal/ml) was purchased from a local store and diluted in water to make 8% (w/v) alcohol. Mice were supplemented with 0 (water) or 8% (w/v) alcohol for 1 or 4 mo (n = 6–8 per experiment, as indicated in the respective figure legends). According to published articles, 8% (w/v) alcohol can be considered as a moderate consumption (27, 28). A liquid diet was not used in this study because alcohol that was provided in the diet would reduce diet intake. The alcohol group had access to water for 6 h every 3 d. Alcohol intake was measured and counted as part of total caloric intake. Genetic recombination was induced by 20 mg/kg body weight tamoxifen injection daily for 3 d, followed by 1 weekly injection of tamoxifen during the dietary trial to induce gene mutation in newly generated PDGFRα+ cells. Wild-type (WT) mice (PDGFRα-Cre−/−/ROSA26-RARαDN+/+) also received tamoxifen injections at the same dose and frequency. At the end of the experiment, mice were unfed for 6 h, then euthanized for serum and adipose tissue sampling.
In the high-fat diet (HFD)-induced obesity trial, control diet (CD; 4.7 IU/g vitamin A, 10% energy from fat, D12450H; Research Diets, New Brunswick, NJ, USA), HFD (4.7 IU/g vitamin A, 60% energy from fat, D12492; Research Diets), and HFD plus 8% (w/v) alcohol in water were fed to mice for 1 mo. In other trials, mice were fed with a commercial rodent diet (15 IU/g vitamin A; 2018 Teklad Global; Harlan, Indianapolis, IN, USA).
To rapidly induce obesity, mice that were used in the HFD trial were 18 wk old. Mice used in other trials were 10 wk old. All mice used in this study were males.
Tissue processing and histology
Adipose tissues were fixed in 4% paraformaldehyde for 12 h at 4°C, then paraffin embedded and sectioned. After deparaffinization, tissue sections were used for hematoxylin and eosin staining or immunostaining. For immunostaining, sections were heated in citrate buffer for 20 min, blocked with 5% goat serum in Tris-buffered saline that contained 0.3% Triton X-100 for 2 h, then incubated sequentially with primary Abs overnight and secondary Abs for 1 h. Sections were then mounted in a mounting medium (Vector Laboratories, Burlingame, CA, USA).
Metabolic chamber analyses
Two days before tissue sample collection, the metabolic rate [Vo2, Vco2, respiratory exchange ratio (RER) and heat production] of mice during the day (quiescent phase) and night (active phase) were measured by using an indirect open circuit calorimetry system [Comprehensive Lab Animal Monitoring System (CLAMS); Columbus Instruments, Columbus, OH, USA]. Mice were unfed for 6 h before measurement and continuously measured for 24 h (with water/alcohol provided), with measurements taken every 30 s (29).
Body temperature measurement
Rectal temperatures were measured by using a TH-5 Thermalert Monitoring Thermometer (Physitemp Instruments, Clifton, NJ, USA). Surface temperatures were measured by using a FLIR E6 thermal imaging camera (FLIR System, Wilsonville, OR, USA).
Glucose and insulin tolerance test
After 6 h unfed, mice received 2 g/kg body weight d-glucose (glucose tolerance test) or 2 U/kg insulin (insulin tolerance test) by i.p. injection. Blood samples were collected from the tail vein at 0, 15, 30, 60, 90, and 120 min postinjection, and glucose concentration was measured by using a glucose meter (Contour; Bayer, Pittsburgh, PA, USA).
HPLC
To prevent isomerization and degradation of retinoids, samples were stored at −80°C before analysis. Samples were protected from light at all times. Retinoids were extracted according to a published protocol (30). Serum and tissue retinoids were measured by HPLC using a reverse-phase column [Luna 3-µm C18(2) 100-Å LC column, 150 × 3 mm; Phenomenex, Torrance, CA, USA] (31). During the mobile phase, methanol/H2O (65/35%) was pumped at 1.0 ml/min. ROL, RA, and retinaldehyde (RAL) were detected by a Nexera ×2 diode array detector (Shimadzu, Kyoto, Japan) at wavelengths of 325, 340, and 385 nm respectively. ROL, RA, and RAL standards were used for calculating standard curves and recovery rates, and their concentrations were expressed as picomoles per milligram wet tissue weight.
Quantitative real-time PCR
Total RNA was isolated by using Trizol (Thermo Fisher Scientific, Waltham, MA, USA), and cDNA was synthesized by using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Real-time quantitative (q)PCR was performed by using the CFX real-time PCR detection system with SYBR Green RT-qPCR Kit (Bio-Rad). Primer sequences are listed in Supplemental Table 1. All values were normalized to the level of a housekeeping gene, 18S rRNA.
Immunoblotting analyses
Immunoblotting analysis were performed as previously described (32) by using an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). Density of bands was quantified, then normalized according to β-tubulin content.
Abs and chemicals
Abs against β-tubulin and ALDH1A1 were purchased from Cell Signaling Technology (Danvers, MA, USA). Abs against UCP1 and PR domain-containing 16 (PRDM16) were purchased from Thermo Fisher Scientific. Tamoxifen was purchased from Sigma-Aldrich (St Louis, MO, USA). Abs were diluted 1:1000.
Serum analysis
Glucose concentrations were measured by using a glucose meter (Contour; Bayer). Serum insulin levels were analyzed by using a Mouse Ultrasensitive Insulin ELISA Kit (Alpco Diagnostics, Salem, NH, USA). Serum triacylglycerols were analyzed by using a triacylglyceride colorimetric assay kit purchased from Cayman Chemical (Ann Arbor, MI, USA). Serum-free fatty acid levels were analyzed by using an EnzyChrom Free Fatty Acid Assay Kit (BioAssay Systems, Hayward, CA, USA).
Statistical analyses
All data are presented as means ± sem. One-way ANOVA followed by post hoc Tukey’s test was used to compare differences between multiple groups. An unpaired, 2-tailed Student’s t test was used to compare differences between 2 groups. All analyses were performed by using SAS (v.9.0; SAS Institute, Cary, NC, USA). Statistical significance was assigned at P < 0.05.
RESULTS
Moderate alcohol intake induces beige adipogenesis in inguinal WAT
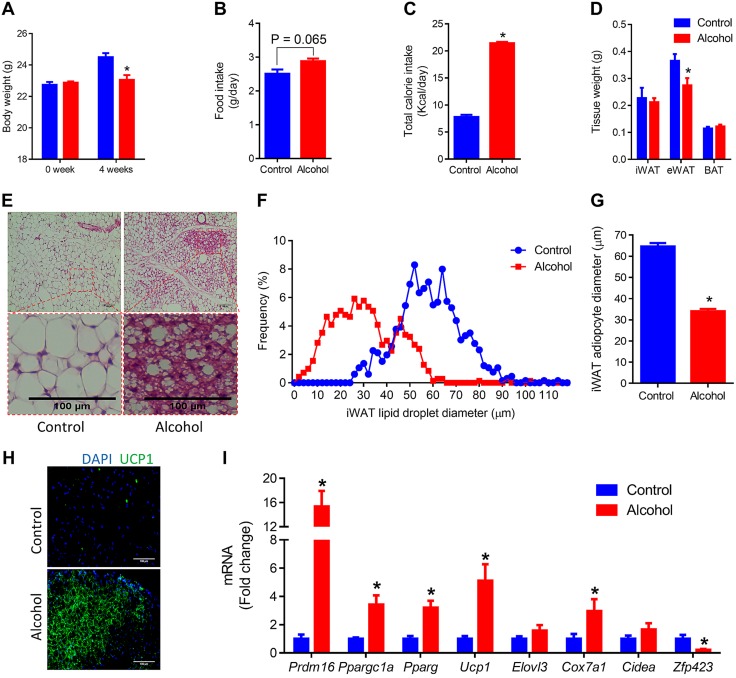
To explore the effects of moderate alcohol intake on the browning of adipose tissue, C57BL6 male mice were supplemented with 0 or 8% (w/v) alcohol in water for 1 mo. Alcohol supplementation dramatically reduced body weight gain (Fig. 1A), although alcohol-supplemented mice tended to have increased food intake (Fig. 1B) and significantly higher total caloric intake, which suggests higher energy expenditure. Alcohol-supplemented mice had lower epidydimal WAT weight (Fig. 1D) and smaller lipid droplets in inguinal WAT (iWAT) (Fig. 1E–G). More UCP1+ cells were observed in iWAT of alcohol-supplemented mice (Fig. 1H). Furthermore, alcohol intake strongly up-regulated the expression of Pdrm16 (Fig. 1I), which is an indispensable transcription factor for committing progenitor cells to the brown/beige adipogenic lineage (33). In addition, alcohol also promoted the expression of peroxisome proliferator-activated receptor-γ coactivator 1α (Ppargc1a) (Fig. 1I), which is another important transcription factor that contributes to beige adipogenesis through the induction of mitochondriogenesis (34). Meanwhile, the required transcription factor for both brown and white adipogenesis (35), peroxisome proliferator-activated receptor-γ (Pparg), was up-regulated by alcohol (Fig. 1I). Moreover, alcohol increased the expression of beige/brown adipose genes, including Ucp1 and cytochrome c oxidase subunit VIIa 1 (Cox7a1), but reduced the expression of white adipogenic gene, Zfp423 (Fig. 1I). Alcohol reduced lipid droplet size (Supplemental Fig. 1A) and up-regulated the expression of brown adipose genes (Supplemental Fig. 1B) in BAT. In summary, these data show that moderate alcohol intake induces beige adipogenesis and reduces fat accumulation.
Figure 1.
Moderate alcohol intake induces beige adipogenesis in iWAT. C57BL6 mice were supplemented with 0 or 8% (w/v) alcohol for 1 mo. A) Body weight. B) Food intake. C) Total caloric intake. D) Adipose tissue weight. E) Representative images of hematoxylin- and eosin-stained iWAT tissue sections. F) Distribution of iWAT lipid droplet diameter. G) Average iWAT adipocyte diameter. H) Representative images of immunohistochemistry of iWAT sections using anti-UCP1 Ab. I) mRNA levels of Prdm16 (PR domain-containing 16), Ppargc1a (peroxisome proliferator-activated receptor-γ coactivator 1 α), Pparg (peroxisome proliferator-activated receptor-γ), Ucp1 (uncoupling protein 1), Elovl3 (elongation of very long chain fatty acids protein 3), Cox7a1 (cytochrome c oxidase subunit VIIa 1), Cidea (cell death-inducing DNA fragmentation factor α-like effector A), and Zfp423 (zinc-finger protein 423) in iWAT. eWAT, epidydimal WAT. *P < 0.05 (n = 8, mean ± sem).
Moderate alcohol intake activates thermogenic activity and improves insulin sensitivity
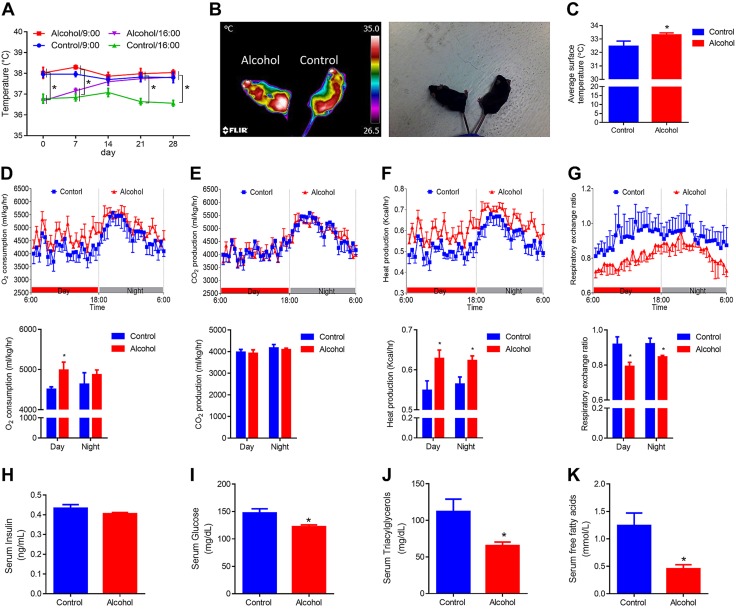
To evaluate the effects of alcohol on thermogenic activity, we measured the rectal temperature of both control and alcohol-supplemented mice on a weekly basis. This was done by taking 2 measurements at 9:00 am and 4:00 pm. There was no rectal temperature difference in the morning, but in the afternoon, the alcohol-supplemented group had higher rectal temperature (Fig. 2A). The lack of difference in the morning could be a result of activity overnight, which could generate heat, masking the difference in basal thermogenesis (36). Conversely, mice were in a quiescent state in the afternoon, depending on basal thermogenesis, to maintain body temperature. In addition, by using a thermal camera, we detected a higher surface temperature in alcohol-supplemented mice (Fig. 2B, C). Metabolic rates of mice were further analyzed. Alcohol-supplemented mice had higher oxygen consumption (Fig. 2D) and heat production (Fig. 2F), which demonstrates elevated metabolic rates. Of interest, alcohol-supplemented mice had a lower RER than control mice (Fig. 2G), which suggests a preferential oxidation of fat vs. carbohydrates.
Figure 2.
Moderate alcohol intake activates thermogenic activity and improves insulin sensitivity to regulate glucose and lipid homeostasis. A) Rectal temperature. B) Representative thermal images. C) Average surface temperature. D) Oxygen consumption. E) CO2 production. F) Heat production. G) RER. H) Serum insulin levels. I) Serum glucose levels. J) Serum triacylglycerol levels. K) Serum free fatty acid levels. *P < 0.05 (n = 8, mean ± sem).
Although no difference in serum insulin levels was observed (Fig. 2H), serum glucose in alcohol-supplemented mice was reduced by ∼35% (Fig. 2I), serum triacylglycerols by ∼40% (Fig. 2J), and serum free fatty acids by ∼60% (Fig. 2K), which suggests that alcohol intake enhances fatty acid and glucose oxidation and is consistent with enhanced beige adipogenesis in these mice.
Effects of 4-mo alcohol intake on adipose tissue browning
To explore the long-term effects of moderate alcohol intake on adipose tissue browning, mice were supplemented with 0 or 8% (w/v) alcohol for 4 mo. Similar to the 1-mo trial, 4-mo alcohol intake reduced body weight gain and increased food intake during the first 3 wk, but reduced food intake during the last 3 wk, which is likely a result of the smaller body size of the alcohol-supplemented group. Consistently, 4-mo alcohol intake reduced white adipose mass and adipocyte size, up-regulated brown adipose genes, and reduced serum concentrations of glucose, triacylglycerols, and free fatty acids (Supplemental Fig. 2).
Moderate alcohol intake increases RA levels in serum and iWAT
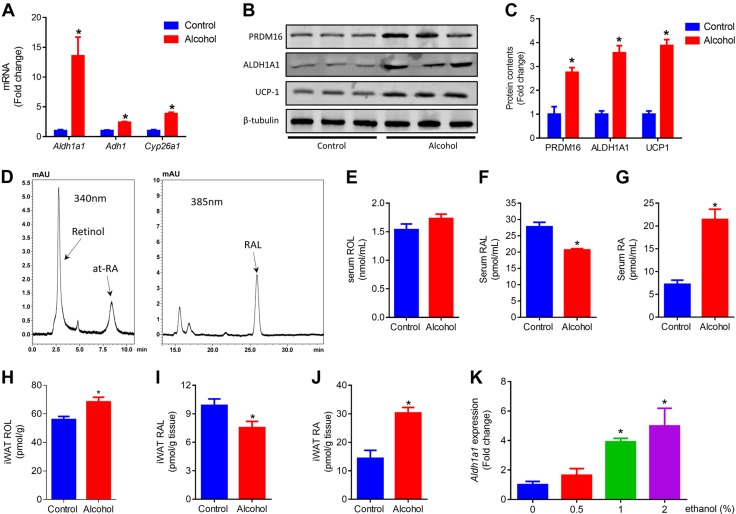
In addition to the liver, WAT and BAT are major sites of retinoid storage (37). Aldehyde dehydrogenase family 1 subfamily A1 (ALDH1A1), the enzyme that is responsible for the oxidation of both aldehyde and RAL, is abundant in adipose tissue (38). Of interest, a marked increase of Aldh1a1 was observed in iWAT of alcohol-supplemented mice (Fig. 3A). The protein content of ALDH1A1 in iWAT of both control and alcohol-supplemented mice was correlated with that of PRDM16 and UCP1 (Fig. 3B, C). Consistently, less RAL (Fig. 3F), but more of its derivative, RA (Fig. 3G), was detected in serum. Alcohol increased ROL (Fig. 3H) and RA (Fig. 3J), but reduced RAL (Fig. 3I) in iWAT. Although higher expressions of the aldehyde-generating enzyme, alcohol dehydrogenase 1 (Adh1), and RA catabolism enzyme, cytochrome P450 26A1 (Cyp26a1), were also found in iWAT of alcohol-supplemented mice (Fig. 3A), ALDH1A1 was the dominant enzyme that controlled RA levels. The effect of ethanol on Aldh1a1 expression was further confirmed in 3T3L1 cells where ethanol up-regulated Aldh1a1 expression dose dependently (Fig. 3K). In addition, alcohol also up-regulated Aldh1a1 and Adh1 in the liver (Supplemental Fig. 1C). Overall, these data show that moderate alcohol intake favors the conversion of vitamin A to RA.
Figure 3.
Moderate alcohol intake increases circulation RA level in serum and iWAT. A) mRNA levels of Aldh1a1 (aldehyde dehydrogenase family 1 subfamily A1), Adh1 (aldehyde dehydrogenase 1), and Cyp26a1 (cytochrome P450 26A1) in iWAT. B) Protein contents of PRDM16, ALDH1A1, and UCP1 in iWAT. C) Quantification of protein contents. D) Representative chromatograms of ROL, all-trans RA (at-RA), and RAL. E) Serum ROL levels. F) Serum RAL levels. G) Serum RA levels. H) iWAT ROL levels. I) iWAT RAL levels. J) iWAT RA levels. K) Expression of Aldh1a1 in 3T3-L1 cells that were treated with ethanol for 24 h. *P < 0.05 (n = 8, mean ± sem).
RA signaling plays an indispensable role in alcohol-induced beige/brown adipogenesis and thermogenesis
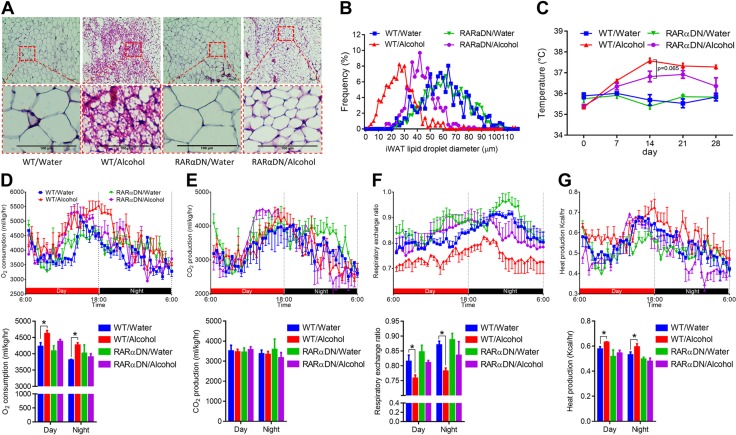
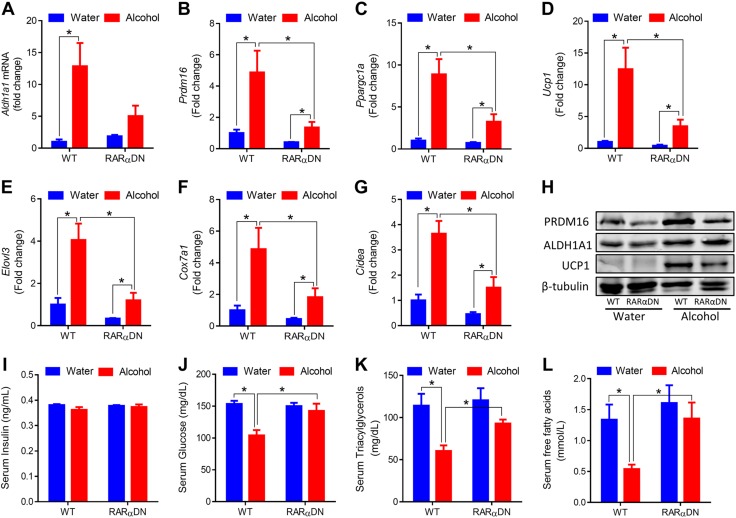
PDGFRα+ cells can differentiate into both beige and white adipocytes (39). Both adipose precursors and mature adipocytes have a history of PDGFRα expression (40). Compared with mature adipocyte markers, such as adiponectin, which is not expressed in stromal vascular cells (40), PDGFRα is a better lineage marker with which to study the differentiation of adipocyte precursors (40, 41). To confirm that alcohol induces beige/brown adipogenesis and thermogenesis via RA signaling, we used ROSA26-RARαDN and PDGFRα-cre/ERT crossed mice. PDGFRα-cre/ERT mice were previously used to trace the adipocyte precursor population (39). When administered with tamoxifen, RARαDN, a dominant-negative RA receptor (RARα403), is expressed that blocks RA signaling in cells that express cre, which, in this case, are PDGFRα+ adipose progenitor cells. We first confirmed that, when fed with water, PDGFRα-cre/ERT+/−RARαDN+/+ mice (RARαDN) had no beige adipocytes (Fig. 4A) and no change in adipocytes size compared with WT mice (PDGFRα-cre/ERT−/−RARαDN+/+; Fig. 4B). Fewer multilocular beige adipocytes were observed in RARαDN/alcohol mice compared with WT/alcohol mice, which shows that RARαDN blocked the browning of WAT (Fig. 4A). The size of lipid droplets in RARαDN/alcohol were smaller than that of WT/water mice but larger than that of WT/alcohol mice (Fig. 4B). Consistent with previous data, alcohol intake increased rectal temperature in WT mice, which was largely absent in RARαDN mice (Fig. 4C). Alcohol increased oxygen consumption in WT mice, but not in RARαDN mice (Fig. 4D), whereas no difference in CO2 production was observed (Fig. 4E), which demonstrates an increase in the oxidation of fatty acids. In line with these results, alcohol reduced RER in WT mice, but not in RARαDN mice (Fig. 4F), and heat production was increased in WT/alcohol mice but not in RARαDN/alcohol mice (Fig. 4G).
Figure 4.
RARα mutation in PDGFRα+ adipose progenitors blocks alcohol effects on adipose tissue browning. RARαDN expression was induced in PDGFRα+ progenitor cells by tamoxifen and supplemented with 8% (w/v) alcohol for 1 mo. PDGFRα-cre/ERT−/−RARαDN+/+; RARαDN: PDGFRα-cre/ERT+/−RARαDN+/+. A) Representative images of hematoxylin- and eosin-stained iWAT tissue sections. B) Distribution of iWAT lipid droplet diameter. C) Rectal temperature measured at 16:00. D) Oxygen consumption. E) CO2 production. F) RER. G) Heat production. *P < 0.05 (n = 6, mean ± sem).
We further analyzed the expression of brown adipose genes in iWAT. Alcohol intake increased the expression of Aldh1a1 in both WT and RARαDN mice (Fig. 5A). Consistent with previous data, alcohol increased the mRNA levels of Prdm16, Ppargc1a, Ucp1, Elovl3 (elongation of very long chain fatty acids protein 3), Cox7a1, and Cidea (cell death-inducing DNA fragmentation factor α-like effector A), and RARαDN prevented these effects of alcohol (Fig. 5B–G), which was consistent with changes in the protein contents of ALDH1A1, PRDM16, and UCP1 (Fig. 5H). Furthermore, RARαDN prevented alcohol-induced reduction in serum glucose (Fig. 5J), triacylglycerols (Fig. 5K), and free fatty acids (Fig. 5L). Overall, these data demonstrate the indispensable role of RA signaling in alcohol-induced beige/brown adipogenesis and thermogenesis.
Figure 5.
RARα mutation in PDGFRα+ adipose progenitors blocks alcohol effects on brown adipogenic gene expression and glucose and lipid homeostasis. A–G) mRNA levels of Aldh1a1 (A), Prdm16 (B), Ppargc1a (C), Ucp1 (D), Elovl3 (E), Cox7a1 (F), and Cidea (G) in iWAT. H) Protein contents of PRDM16, ALDH1A1, and UCP1 in iWAT. I) Serum insulin levels. J) Serum glucose levels. K) Serum triacylglycerol levels. L) Serum free fatty acid levels. *P < 0.05 (n = 6, mean ± sem).
Moderate alcohol intake prevents obesity in a mouse model of diet-induced obesity
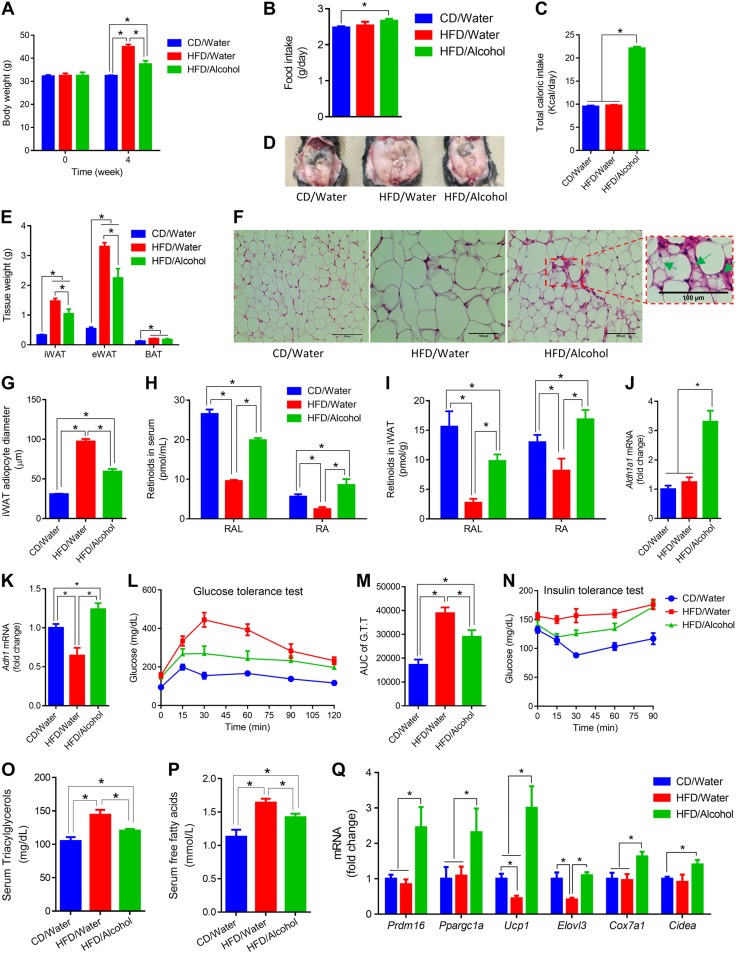
The effects of alcohol on adipose tissue were further explored in a HFD-induced obesity model. When challenged with HFD, compared with water group, alcohol-supplemented mice had lower body weight gain (Fig. 6A), although alcohol-supplemented mice had higher food intake (Fig. 6B) and total caloric intake (Fig. 6C), which suggests increased energy expenditure in alcohol-supplemented mice. Consistently, alcohol reduced white fat mass, which was increased by HFD (Fig. 6D, E). Alcohol prevented the increase of adipocyte size of HFD-fed mice (Fig. 6F, G). Multilocular beige adipocytes were observed in iWAT of alcohol-supplemented mice (Fig. 6F). HFD reduced RAL and RA levels in both serum and iWAT in the absence of alcohol consumption (Fig. 6H, I). RAL levels in serum and iWAT of HFD/alcohol mice were lower than CD/water mice, but higher than that of HFD/water mice (Fig. 6H, I). HFD/alcohol mice had the highest RA levels in serum and iWAT (Fig. 6H, I). Consistently, alcohol up-regulated Aldh1a1 (Fig. 6J) and Adh1 (Fig. 6K) in iWAT and HFD down-regulated Adh1 (Fig. 6K). HFD-treated mice developed glucose intolerance (Fig. 6L, M) and insulin resistance (Fig. 6N). Alcohol improved glucose tolerance (Fig. 6L, M) and insulin sensitivity (Fig. 6N). Consistently, alcohol reduced circulatory triacylglyceride (Fig. 6O) and free fatty acid (Fig. 6P) levels when challenged with HFD. Moreover, alcohol increased the expression of Prdm16, Ppargc1a, Ucp1, Elovl3, Cox7a1, and Cidea (Fig. 6Q).
Figure 6.
Moderate alcohol intake induces beige adipogenesis and prevents obesity in a mouse model of diet-induced obesity. C57BL6 male mice were fed CD (CD/water), HFD (HFD/water), and HFD plus 8% (w/v) alcohol (HFD/alcohol) for 1 mo. A) Body weight. B) Food intake. C) Total caloric intake. D) Representative images showing WAT. E) Adipose tissues weight. F) Representative images of iWAT (green arrows indicate beige adipocytes). G) Average iWAT adipocyte diameter. H) RAL and RA in serum. I) RAL and RA in iWAT. J) Aldh1a1 expression in iWAT. K) Adh1 expression in iWAT. L) Glucose tolerance test. M) AUC of glucose tolerance test (G.T.T). N) Insulin tolerance test. O) Serum triacylglycerol levels. P) Serum free fatty acid levels. Q) iWAT mRNA levels. *P < 0.05 (n = 8, mean ± sem).
DISCUSSION
Because alcohol energy is additive to the diet and moderate alcohol intake increases caloric intake (8), alcohol intake has been regarded as contributing to obesity. Although several studies have reported a positive association between alcohol consumption and obesity (42), especially among heavy alcohol drinkers (43), evidence from epidemiologic studies suggests that moderate alcohol intake surprisingly reduces the risk of obesity (8, 10, 44). In fact, moderate alcohol intake demonstrates beneficial effects on the prevention of obesity (10, 44), diabetes (16), and fatty liver disease (14, 15). Here, we consistently found that moderate alcohol intake increased caloric intake but reduced body weight by increasing energy expenditure and thermogenesis, which explains the beneficial effects of alcohol intake on glucose and lipid clearance (17), and improving insulin sensitivity (18).
Metabolism of vitamin A follows a parallel pathway of ethanol metabolism, and the oxidative enzymes, alcohol dehydrogenase and aldehyde dehydrogenase, can catalyze both ethanol and vitamin A oxidation (19). Studies have shown that alcohol increases ALDH enzyme activity (45, 46), ALDH1A1 is the major RA-generating enzyme in adipose (38), which is also an important enzyme for alcohol metabolism (47). Short-term ethanol supplement increased RA in adult tissues (20), in cultured cerebellar astrocytes, and in the cerebellum (48). We found that alcohol intake strongly increased RA levels in serum and adipose tissue by increasing ALDH1A1. Vitamin A and RA are known to reduce obesity risks. RA inhibits white adipogenesis by up-regulating preadipocyte genes (49). In contrast, RA induces UCP1 expression (50), thereby suppressing obesity by promoting energy expenditure (24, 51). In this study, we found that alcohol elevated RA levels, which induce beige adipogenesis and thermogenesis, effectively reducing obesity and metabolic dysfunction in mice. A recent study reported that alcohol consumption decreased BAT weight and altered thermogenesis (52). From the data presented, BAT weight was reduced, most likely as a result of reduced lipid content. Alcohol-supplemented mice had higher body temperatures during the light cycle, which indicated higher basal metabolic rate, and the lower body temperature during the dark cycle may be a result of alcohol-induced inactivity. The overall data are consistent with the current study; however, because mice consume less alcohol liquid diet than CD (53), an ad libitum feeding protocol would lead to altered food and vitamin A intake. This may explain the different observations of retinoid concentration in their study.
Overexpression of dominant-negative RAR mutant is a widely used method for blocking RAR function (26). PDGFRα+ cells are adipose progenitors that can differentiate into both brown and white adipocytes upon activation of β3-adrenergic signaling or with an HFD (39). This population has been identified as an adipogenic niche for adipose tissue remodeling (54). To establish the necessity of RAR-mediated signaling in beige adipogenesis induced by alcohol, we specifically overexpressed RARαDN in PDGFRα+ adipose progenitor cells, which blocked RAR signaling. Indeed, blocking RAR signaling largely abolished the effect of alcohol on the browning of iWAT, which showed that alcohol induces de novo beige adipogenesis and enhances energy dissipation via elevation of RAR signaling.
We previously reported that lack of RA signaling impairs fetal adipose development (31). ALDH1A1 permanent knockout mice are resistant to diet-induced obesity (55, 56) because ALDH1A1 deficiency mice had almost no fat because of impaired adipogenesis at fetal stage (55); however, as demonstrated in Pparg (57) and Zfp423 (41) deletion mice, although mice became leaner, they showed metabolic disorders because of ectopic accumulation of lipids in liver and muscle with a lack of adipocytes.
Overweight and obesity are associated with vitamin A deficiency (58, 59). HFD suppresses retinol dehydrogenase activity, which is consistent with a previous study (60). In the current study, HFD dramatically reduced RAL levels in serum and iWAT. Although no difference was observed in Aldh1a1 expression, HFD in the absence of alcohol strongly reduced RA in serum and iWAT, which should be a result of RAL deficiency.
Although moderate alcohol intake benefits glucose and lipid homeostasis by elevating RA levels, long-term excessive alcohol intake may lead to vitamin A deficiency. As previously reviewed (19), alcoholics have reduced ROL levels and plasma retinol binding protein concentrations. Chronic alcoholism is associated with blindness and liver diseases that are caused by vitamin A deficiency (61), which may explain higher body mass index in persons with heavy alcohol intake (43). Deficiency in vitamin A renders it impossible to increase RA signaling and thus browning of WAT. The liver is the major site of vitamin A storage and chronic excessive alcohol intake dramatically reduces hepatic retinoid content (21) and leads to fatty liver (62). As a result, for chronic alcoholics, supplementation with vitamin A to prevent a deficiency may be critically important for metabolic health. The interplay of alcohol consumption and vitamin A turnover in determining the outcomes of alcohol consumption on obesity and metabolic health requires additional exploration.
In summary, moderate alcohol intake induces WAT browning and enhances thermogenesis and energy dissipation via an RA-dependent mechanism. This newly discovered mechanism provides insight into the preventive effects of moderate alcohol intake on obesity and metabolic dysfunction.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was funded by U.S. National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01-HD067449, NIH National Institute on Aging Grant R21-AG049976, and U.S. Department of Agriculture, National Institute of Food and Agriculture Award 2015-67015-23219). The authors thank Dr. Cathy Mendelsohn (Columbia University, New York, NY, USA) for providing ROSA26-RARαDN mice. The authors declare no conflicts of interest.
Glossary
- ADH1
aldehyde dehydrogenase 1
- ALDH1A1
aldehyde dehydrogenase family 1 subfamily A1
- BAT
brown adipose tissue
- CD
control diet
- Cidea
cell death-inducing DNA fragmentation factor α-like effector A
- Cox7a1
cytochrome c oxidase subunit VIIa 1
- Cyp26a1
cytochrome P450 26A1
- Elovl3
elongation of very long chain fatty acids protein 3
- HFD
high-fat diet
- iWAT
inguinal white adipose tissue
- PDGFR
platelet-derived growth factor receptor
- Pparg
peroxisome proliferator-activated receptor-γ
- Ppargc1a
peroxisome proliferator-activated receptor-γ coactivator 1 α
- PRDM16
PR domain-containing 16
- RA
retinoic acid
- RAL
retinaldehyde
- RAR
retinoic acid receptor
- RER
respiratory exchange rate
- ROL
retinol
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
- WT
wild type
- Zfp423
zinc-finger protein 423
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. Wang and M. Du conceived the project, designed the experiments, and wrote the manuscript; B. Wang, Z. Wang, J. M. de Avila, N. A. Gomez, L. Zhao, Q. Tian, and J. Zhao performed the experiments; F. Zhang and H. Zhang performed mouse treatments for the preliminary experiments; J. Maricelli and B. D. Rodgers assisted in metabolic chamber analyses; and M.-J. Zhu contributed to the discussion and reviewed and edited the manuscript.
REFERENCES
- 1.King B. M. (2013) The modern obesity epidemic, ancestral hunter-gatherers, and the sensory/reward control of food intake. Am. Psychol. 68, 88–96 [DOI] [PubMed] [Google Scholar]
- 2.Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W. D., Hoeks J., Enerbäck S., Schrauwen P., and Spiegelman B. M. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon B., and Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 4.Tseng Y.-H., Cypess A. M., and Kahn C. R. (2010) Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 9, 465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., and Nuutila P. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 6.Lee P., Werner C. D., Kebebew E., and Celi F. S. (2014) Functional thermogenic beige adipogenesis is inducible in human neck fat. Int. J. Obes. (Lond.) 38, 170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peschechera A., and Eckel J. (2013) “Browning” of adipose tissue--regulation and therapeutic perspectives. Arch. Physiol. Biochem. 119, 151–160 [DOI] [PubMed] [Google Scholar]
- 8.Yeomans M. R. (2010) Alcohol, appetite and energy balance: is alcohol intake a risk factor for obesity? Physiol. Behav. 100, 82–89 [DOI] [PubMed] [Google Scholar]
- 9.De Castro J. M., and Orozco S. (1990) Moderate alcohol intake and spontaneous eating patterns of humans: evidence of unregulated supplementation. Am. J. Clin. Nutr. 52, 246–253 [DOI] [PubMed] [Google Scholar]
- 10.Liu S., Serdula M. K., Williamson D. F., Mokdad A. H., and Byers T. (1994) A prospective study of alcohol intake and change in body weight among US adults. Am. J. Epidemiol. 140, 912–920 [DOI] [PubMed] [Google Scholar]
- 11.Cordain L., Bryan E. D., Melby C. L., and Smith M. J. (1997) Influence of moderate daily wine consumption on body weight regulation and metabolism in healthy free-living males. J. Am. Coll. Nutr. 16, 134–139 [DOI] [PubMed] [Google Scholar]
- 12.Arif A. A., and Rohrer J. E. (2005) Patterns of alcohol drinking and its association with obesity: data from the Third National Health and Nutrition Examination Survey, 1988-1994. BMC Public Health 5, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrières J. (2004) The French paradox: lessons for other countries. Heart 90, 107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriya A., Iwasaki Y., Ohguchi S., Kayashima E., Mitsumune T., Taniguchi H., Ikeda F., Shiratori Y., and Yamamoto K. (2011) Alcohol consumption appears to protect against non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 33, 378–388 [DOI] [PubMed] [Google Scholar]
- 15.Moriya A., Iwasaki Y., Ohguchi S., Kayashima E., Mitsumune T., Ikeda F., Ando M., and Yamamoto K. (2013) Roles of alcohol drinking pattern in fatty liver in Japanese women. Hepatol. Int. 7, 859–868 [DOI] [PubMed] [Google Scholar]
- 16.Koppes L. L., Dekker J. M., Hendriks H. F., Bouter L. M., and Heine R. J. (2005) Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 28, 719–725 [DOI] [PubMed] [Google Scholar]
- 17.Joosten M. M., de Graaf C., Rietman A., Witkamp R. F., and Hendriks H. F. (2010) Short-term oral exposure to white wine transiently lowers serum free fatty acids. Appetite 55, 124–129 [DOI] [PubMed] [Google Scholar]
- 18.Avogaro A., Watanabe R. M., Dall’Arche A., De Kreutzenberg S. V., Tiengo A., and Pacini G. (2004) Acute alcohol consumption improves insulin action without affecting insulin secretion in type 2 diabetic subjects. Diabetes Care 27, 1369–1374 [DOI] [PubMed] [Google Scholar]
- 19.Clugston R. D., and Blaner W. S. (2012) The adverse effects of alcohol on vitamin A metabolism. Nutrients 4, 356–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane M. A., Folias A. E., Wang C., and Napoli J. L. (2010) Ethanol elevates physiological all-trans-retinoic acid levels in select loci through altering retinoid metabolism in multiple loci: a potential mechanism of ethanol toxicity. FASEB J. 24, 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leo M. A., and Lieber C. S. (1982) Hepatic vitamin A depletion in alcoholic liver injury. N. Engl. J. Med. 307, 597–601 [DOI] [PubMed] [Google Scholar]
- 22.Dani C., Smith A. G., Dessolin S., Leroy P., Staccini L., Villageois P., Darimont C., and Ailhaud G. (1997) Differentiation of embryonic stem cells into adipocytes in vitro. J. Cell Sci. 110, 1279–1285 [DOI] [PubMed] [Google Scholar]
- 23.Wang B., Fu X., Zhu M.-j., and Du M. (2017) Retinoic acid inhibits white adipogenesis by disrupting GADD45A mediated Zfp423 DNA demethylation. J. Mol. Cell Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noy N. (2013) The one-two punch: retinoic acid suppresses obesity both by promoting energy expenditure and by inhibiting adipogenesis. Adipocyte 2, 184–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercader J., Ribot J., Murano I., Felipe F., Cinti S., Bonet M. L., and Palou A. (2006) Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 147, 5325–5332 [DOI] [PubMed] [Google Scholar]
- 26.Rosselot C., Spraggon L., Chia I., Batourina E., Riccio P., Lu B., Niederreither K., Dolle P., Duester G., Chambon P., Costantini F., Gilbert T., Molotkov A., and Mendelsohn C. (2010) Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137, 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou H. Z., Karliner J. S., and Gray M. O. (2002) Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. Am. J. Physiol. Heart Circ. Physiol. 283, H165–H174 [DOI] [PubMed] [Google Scholar]
- 28.Fan F., Cao Q., Wang C., Ma X., Shen C., Liu X. W., Bu L. P., Zou Y. Z., Hu K., Sun A. J., and Ge J. B. (2014) Impact of chronic low to moderate alcohol consumption on blood lipid and heart energy profile in acetaldehyde dehydrogenase 2-deficient mice. Acta Pharmacol. Sin. 35, 1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S., Liang X., Yang Q., Fu X., Rogers C. J., Zhu M., Rodgers B. D., Jiang Q., Dodson M. V., and Du M. (2015) Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 39, 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y.-K., and Quadro L. (2010) Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol. Biol. 652, 263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B., Fu X., Liang X., Wang Z., Yang Q., Zou T., Nie W., Zhao J., Gao P., Zhu M. J., de Avila J. M., Maricelli J., Rodgers B. D., and Du M. (2017) Maternal retinoids increase PDGFRα+ progenitor population and beige adipogenesis in progeny by stimulating vascular development. EBioMedicine 18, 288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B., Yang G., Liang X., Zhu M., and Du M. (2014) Grape seed extract prevents skeletal muscle wasting in interleukin 10 knockout mice. BMC Complement. Altern. Med. 14, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., Tempst P., Rudnicki M. A., Beier D. R., and Spiegelman B. M. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puigserver P., Wu Z., Park C. W., Graves R., Wright M., and Spiegelman B. M. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 [DOI] [PubMed] [Google Scholar]
- 35.Lowe C. E., O’Rahilly S., and Rochford J. J. (2011) Adipogenesis at a glance. J. Cell Sci. 124, 2681–2686 [DOI] [PubMed] [Google Scholar]
- 36.Ekblom B., and Björntorp P. (2001) Energy expenditure at rest and during exercise. In International Textbook of Obesity (Björntorp P., ed.), John Wiley & Sons Ltd, London [Google Scholar]
- 37.Villarroya F., Giralt M., and Iglesias R. (1999) Retinoids and adipose tissues: metabolism, cell differentiation and gene expression. Int. J. Obes. Relat. Metab. Disord. 23, 1–6 [DOI] [PubMed] [Google Scholar]
- 38.Reichert B., Yasmeen R., Jeyakumar S. M., Yang F., Thomou T., Alder H., Duester G., Maiseyeu A., Mihai G., Harrison E. H., Rajagopalan S., Kirkland J. L., and Ziouzenkova O. (2011) Concerted action of aldehyde dehydrogenases influences depot-specific fat formation. Mol. Endocrinol. 25, 799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y. H., Petkova A. P., Mottillo E. P., and Granneman J. G. (2012) In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry R., and Rodeheffer M. S. (2013) Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao M., Hepler C., Vishvanath L., MacPherson K. A., Busbuso N. C., and Gupta R. K. (2016) Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol. Metab. 6, 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shelton N. J., and Knott C. S. (2014) Association between alcohol calorie intake and overweight and obesity in English adults. Am. J. Public Health 104, 629–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wannamethee S. G., and Shaper A. G. (2003) Alcohol, body weight, and weight gain in middle-aged men. Am. J. Clin. Nutr. 77, 1312–1317 [DOI] [PubMed] [Google Scholar]
- 44.Jéquier E. (1999) Alcohol intake and body weight: a paradox. Am. J. Clin. Nutr. 69, 173–174 [DOI] [PubMed] [Google Scholar]
- 45.Tian L., Deshmukh A., Prasad N., and Jang Y. Y. (2016) Alcohol increases liver progenitor populations and induces disease phenotypes in human IPSC-derived mature stage hepatic cells. Int. J. Biol. Sci. 12, 1052–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Z., Wang L., Song Z., Saari J. T., McClain C. J., and Kang Y. J. (2005) Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am. J. Pathol. 166, 1681–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lind P. A., Eriksson C. J., and Wilhelmsen K. C. (2008) The role of aldehyde dehydrogenase-1 (ALDH1A1) polymorphisms in harmful alcohol consumption in a Finnish population. Hum. Genomics 3, 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCaffery P., Koul O., Smith D., Napoli J. L., Chen N., and Ullman M. D. (2004) Ethanol increases retinoic acid production in cerebellar astrocytes and in cerebellum. Brain Res. Dev. Brain Res. 153, 233–241 [DOI] [PubMed] [Google Scholar]
- 49.Berry D. C., DeSantis D., Soltanian H., Croniger C. M., and Noy N. (2012) Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes 61, 1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puigserver P., Vázquez F., Bonet M. L., Picó C., and Palou A. (1996) In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem. J. 317, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonet M. L., Ribot J., Felipe F., and Palou A. (2003) Vitamin A and the regulation of fat reserves. Cell. Mol. Life Sci. 60, 1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blaner W. S., Gao M. A., Jiang H., Dalmer T. R., Hu X. J., Ginsberg H. N., and Clugston R. D. (2017) Chronic alcohol consumption decreases brown adipose tissue mass and disrupts thermoregulation: a possible role for altered retinoid signaling. Sci. Rep. 7, 43474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertola A., Mathews S., Ki S. H., Wang H., and Gao B. (2013) Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 8, 627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee Y. H., Petkova A. P., and Granneman J. G. (2013) Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 18, 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziouzenkova O., Orasanu G., Sharlach M., Akiyama T. E., Berger J. P., Viereck J., Hamilton J. A., Tang G., Dolnikowski G. G., Vogel S., Duester G., and Plutzky J. (2007) Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 13, 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiefer F. W., Vernochet C., O’Brien P., Spoerl S., Brown J. D., Nallamshetty S., Zeyda M., Stulnig T. M., Cohen D. E., Kahn C. R., and Plutzky J. (2012) Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat. Med. 18, 918–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F., Mullican S. E., DiSpirito J. R., Peed L. C., and Lazar M. A. (2013) Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc. Natl. Acad. Sci. USA 110, 18656–18661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Souza Valente da Silva L., Valeria da Veiga G., and Ramalho R. A. (2007) Association of serum concentrations of retinol and carotenoids with overweight in children and adolescents. Nutrition 23, 392–397 [DOI] [PubMed] [Google Scholar]
- 59.Pereira S. E., Saboya C. J., Saunders C., and Ramalho A. (2012) Serum levels and liver store of retinol and their association with night blindness in individuals with class III obesity. Obes. Surg. 22, 602–608 [DOI] [PubMed] [Google Scholar]
- 60.Zhang M., Liu C., Hu M. Y., Zhang J., Xu P., Li F., Zhong Z. Y., Liu L., and Liu X. D. (2015) High-fat diet enhanced retinal dehydrogenase activity, but suppressed retinol dehydrogenase activity in liver of rats. J. Pharmacol. Sci. 127, 430–438 [DOI] [PubMed] [Google Scholar]
- 61.Halsted C. H. (2004) Nutrition and alcoholic liver disease. Semin. Liver Dis. 24, 289–304 [DOI] [PubMed] [Google Scholar]
- 62.Purohit V., Gao B., and Song B. J. (2009) Molecular mechanisms of alcoholic fatty liver. Alcohol. Clin. Exp. Res. 33, 191–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.