Figure 1.
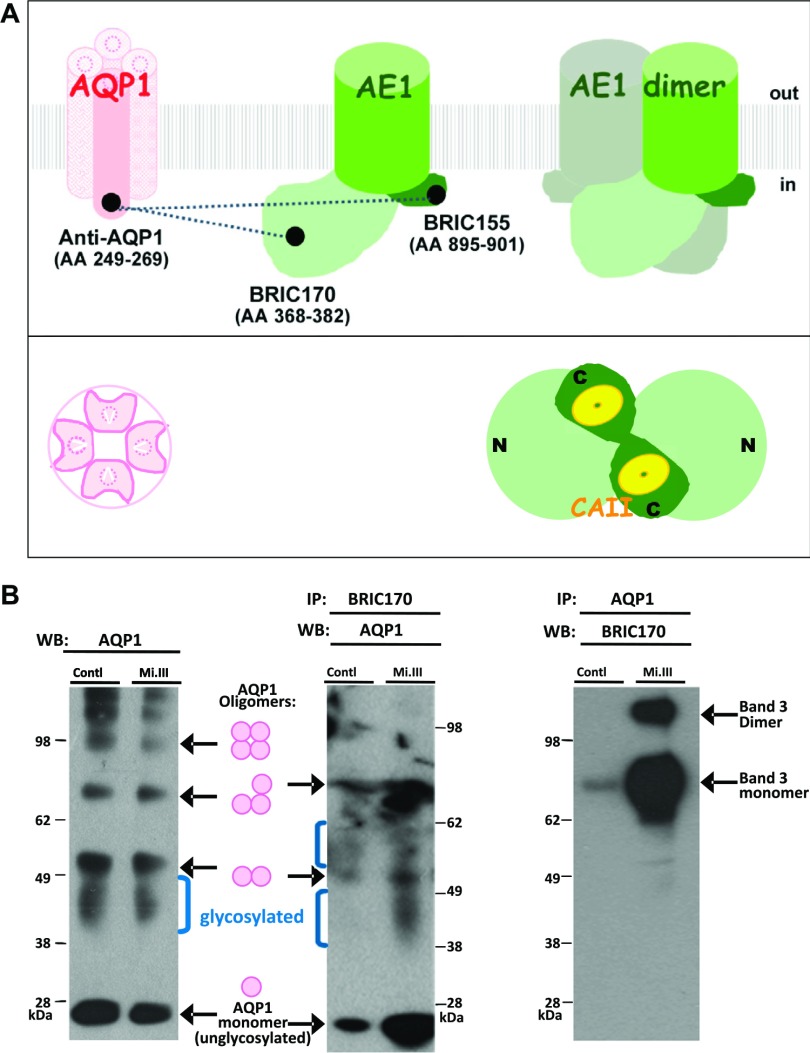
Protein–protein interaction between AQP1 and band 3 in human RBCs. A) A simple illustration of AQP1 and band 3 viewed from the transmembrane cross-section (side view) and from inside an erythrocyte (bottom view). mAbs used in this study are labeled, with their corresponding amino acid epitopes indicated in parentheses. In the band 3 monomer, light green marks the N-terminal cytoplasmic domain of band 3 (aa 1–408), including a flexible linker structure (aa 357–408) connecting to the transmembrane domain; dark green: the short, C-terminal, cytoplasmic region of band 3 (27–29). The transmembrane domain of band 3 (green cylinder), responsible for anion conductance, connects the N- and C-terminal cytoplasmic regions. Dotted lines connecting the epitopes of AQP1 and band 3 represent the molecular interactions tested by FLIM. The bottom view shows an AQP1 tetramer and a band 3 dimer. Although AQP1 exists as a homotetramer, each AQP1 monomer contains a passage for water (30). On the other hand, the Cl−/HCO3− bidirectional transport requires dimeric composition of AE1. N, N-terminal; C, C-terminal cytoplasmic domains of band 3. Yellow: CAII, binding the C-terminal, cytoplasmic region of band 3. B) Co-IP experiments demonstrated stronger AQP1-band 3 interaction in Mi.III than in non-Mi.III (control) erythrocyte membrane. Each group (control/Mi.III) contained mixed ghost samples from 6 individual donors. Left: Western blot analysis with an anti-AQP1 mAb (clone 1/A5F6) showed similar expression levels of AQP in Mi.III and non-Mi.III erythrocytes. Non- and glycosylated AQP1 monomer, dimer, trimer, and tetramer (AQP1 oligomers) were labeled in an AQP1 Western blot. The molecular mass of the nonglycosylated AQP1 monomer was ∼26 kDa, and the molecular masses of glycosylated AQP1 monomers ranged from 34 to 40 kDa (31–33). Middle: co-IP, with anti-band 3 mAb (BRIC170), pulled down much more AQP1 in Mi.III ghosts than in non-Mi.III ghosts. Right: Co-IP by anti-AQP1 mAb pulled down much more band 3 protein in the Mi.III ghosts, compared to the control.