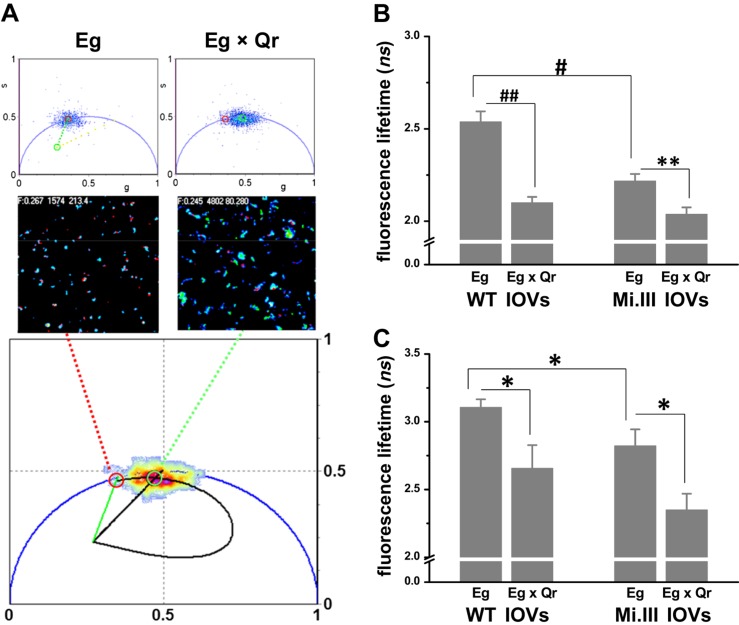
Figure 3.
FLIM-FRET revealed molecular interaction between AQP1 and band 3 on the RBC membrane. BRIC170-Alexa Fluor 488 conjugate labeled N-terminal band 3/AE1 with green fluorescence (Eg; the FRET donor). Anti-AQP1-Alexa Fluor 568 conjugate labeled AQP1 with red fluorescence (Qr; the FRET acceptor). A) Top: the 2 phasor plots showed fluorescence lifetimes (τ) of Alexa Fluor 488 emitted from IOVs, singly labeled with FRET donor Eg or doubly labeled with FRET donor and acceptor (Eg × Qr). Middle: their lifetime images. The bottom phasor plot estimated the degree of energy transfer from Eg to Qr using a trajectory line crossing Eg (red circle; 2.7 ns) to Eg × Qr (green circle; 2.0 ns) (36). B) FLIM-FRET experiments using BRIC170-Alexa Fluor 488 conjugate as the FRET donor demonstrated AQP1–band 3 interaction. Significant decreases in fluorescence lifetimes of Alexa Fluor 488 from Eg singly labeled to Eg × Qr doubly labeled IOVs indicated the occurrence of FRET. Mi.III IOVs showed FRET, but to a lesser degree than WT IOVs. Each group was compiled from 13 to 27 independent sample measurements (mean ± sem). C) FLIM-FRET experiments using BRIC155-Alexa Fluor 488 conjugated as the FRET donor also showed AQP1–band 3 interaction. Eg represents labeling of band 3 with BRIC155-Alexa Fluor 488 conjugate; Qr represents labeling with the same probe. Significant differences in the lifetimes of Alexa Fluor 488 between Eg and Eg × Qr were again observed. Their lifetime differences were similar in both Mi.III and non-Mi.III IOVs. Each group was compiled from 7 to 15 samples. *P < 0.05, **P < 0.005; #P < 0.0001, ##P < 10−6.