Figure 3.
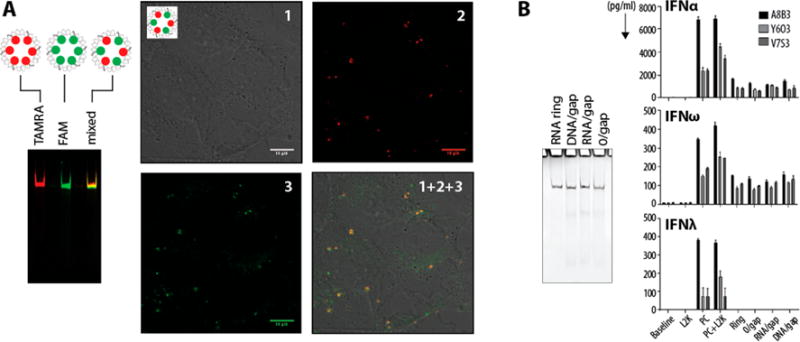
Cell culture experiments. (A) Ring assemblies with either a red 3′-TAMRA-RNA or a green 5′-FAM RNA gap complement or an alternating combination of both (denoted as mixed). The latter is used to confirm the structural integrity of rings during the intracellular colocalization in human breast cancer cells (MDA-MB-231). (B) Immunological studies were conducted in vitro using PBMC derived from three healthy donors (A8B3, Y6O3, and V7S3). ODN2216, a known potent inducer of type I IFNs, was used as the assay positive control (PC). Untreated cells were used to establish a baseline. Lipofectamine (L2K) at the same concentration as that used for the delivery of the RNA nanoparticles was used as a negative control. Both ODN2216 and L2K were also tested together to rule out any negative effects on cell viability and IFN induction when both the delivery vehicle and the IFN inducing sample are added to cells together. Nanoparticles with various modifications (ring, 0/gap, RNA/gap, and DNA/gap) were delivered using lipofectamine. Type I IFN (IFNα and IFNβ) and type III IFN (IFNλ) were analyzed in the supernatants 24 h after the addition of test samples and controls to PBMC cultures. Each bar represents a mean and a standard deviation (N = 3). No difference between nanoparticles with various modifications was identified by this test. All ring assemblies were confirmed by native-PAGE.
