Abstract
Drug survival of biologics represents their real-world effectiveness and safety. We conducted a meta-analysis of real-world evidence on the drug survival of biologics in treating psoriasis. We searched the PubMed, CENTRAL, and EMBASE databases from inception to 7th October 2017 for studies reporting the annual drug survival for at least 1 year. Two authors independently screened and selected relevant studies, and assessed their risk of bias. A third author was available for arbitrating discrepancies. We conducted a random-effects model meta-analysis to obtain the respective pooled drug survival from year 1 to 4. We conducted subgroup analysis on biologic-naïve subjects, discontinuation for loss of efficacy and adverse effects. We included 37 studies with 32,631 subjects. The drug survival for all biologics decreased with time, dropping from 66% at year 1 to 41% at year 4 for etanercept, from 69% to 47% for adalimumab, from 61% to 42% for infliximab, and from 82% to 56% for ustekinumab. Ustekinumab was associated with the highest drug survival in all and biologic-naïve subjects. Etanercept was associated with the lowest drug survival and was most commonly discontinued for loss of efficacy. Infliximab was most frequently associated with discontinuation for adverse effects. Clinicians may use this study as a reference in treating psoriasis.
Introduction
Psoriasis is a chronic inflammatory dermatosis1,2. Biologics are indicated for treating moderate to severe psoriasis that is unresponsive to conventional systemic therapies or phototherapy or when the patients are intolerant to traditional treatments3,4. A recent network meta-analysis has demonstrated excellent short-term efficacy of biologics, including tumor necrosis factor inhibitors (for example etanercept, infliximab, and adalimumab), interleukin (IL)−12/23 inhibitors (such as ustekinumab), IL-17 inhibitors (including secukinumab, ixekizumab, and brodalumab), and IL-23 inhibitors (such as guselkumab and tildrakizumab)5. However, the efficacy of biologics in treating psoriasis usually fades with time. Drug survival, also known as ‘drug retention’ or ‘drug persistence’, is the rate and duration of adherence to biologics, which represent the long-term effectiveness and safety of the biologics in the real world. Drug survival is a useful reference for choosing a biologic for treating psoriasis.
To date there are only individual studies on the drug survival of biologics for treating psoriasis. No et al.6 conducted a systemic review of biologic agents for psoriasis and revealed ustekinumab was associated with the longest median drug survival (38.0 months), which was longer than those of infliximab, adalimumab, and etanercept. However, they did not assess the safety data nor analyze the drug survival in biologic-naïve subjects. To provide physicians with a reference for administering biologics for various clinical settings, we conducted a systematic review and meta-analysis of real-world evidence on the drug survival of biologics in treating psoriasis, and conducted subgroup analyses on biologic-naïve subjects, discontinuation due to loss of efficacy and adverse effects.
Results
Identification and characteristics of studies
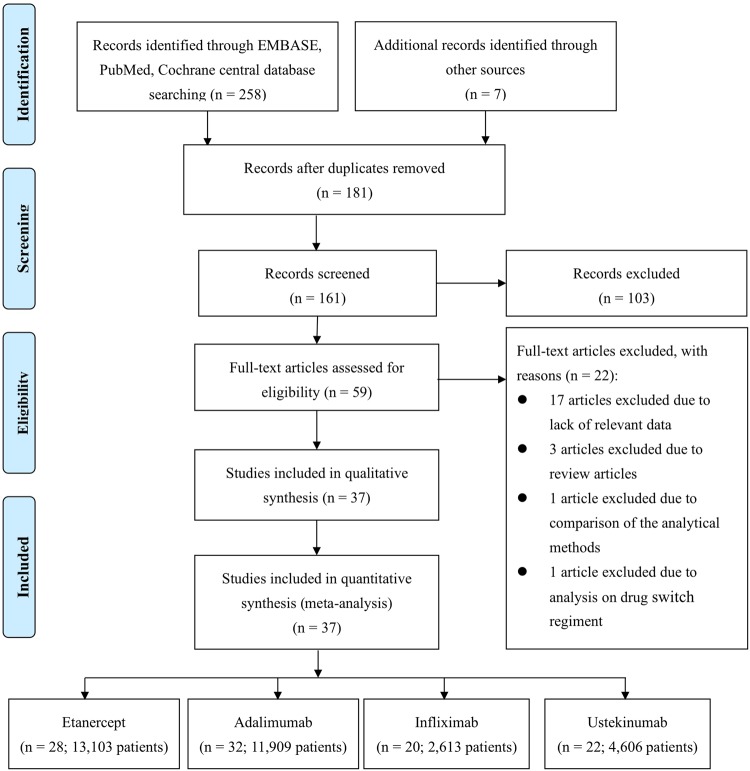
The PRISMA7 study flow diagram is shown in Fig. 1. Our search identified 181 articles after removing 84 duplicates. A total of 37 studies with 32,231 subjects met our inclusion criteria and were included. Six included studies provided usable data for the meta-analysis on discontinuation due to ineffectiveness, while five included studies were available for the meta-analysis on discontinuation due to adverse effects.
Figure 1.
The PRISMA study flow diagram. Adapted from the PRISMA template7.
As to the risk of bias, two studies were rated five stars, twelve of the studies were rated six stars, nineteen of the studies were rated seven, and four were rated eight stars on the Newcastle–Ottawa Scale (NOS)8.
Drug survival of biologics for treating psoriasis
The drug survival for each biologic and the status of patients being biologic-naïve or -experienced are shown in Supplementary Table S1. There were 28 studies9–36 (13,303 patients) reporting data for etanercept, 32 studies9–11,13–19,21–31,33–43 (12,109 patients) for adalimumab, 20 studies9,11,13–19,21–24,26,27,29,30,34,35,40 (2,613 patients) for infliximab, and 22 studies9,13,14,16,19,22–31,33–36,40,44,45 (4,606 patients) for ustekinumab.
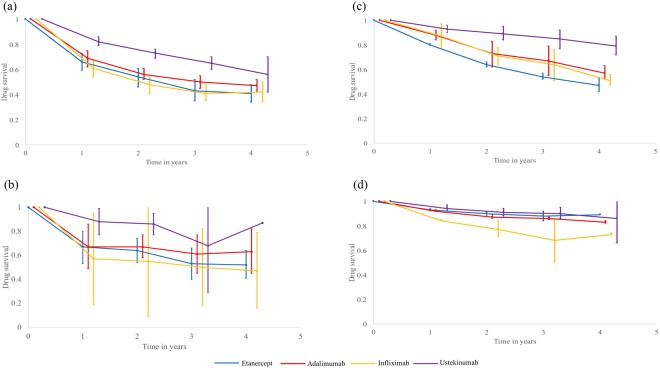
The meta-analysis on the drug survival of each biologic is show in Fig. 2(a), with the forest plots shown in the Supplementary Fig. S1. The drug survival for all biologics decreased year by year. The drug survival rate for etanercept was 1 (i.e. 100%) at year 0 (baseline) and dropped to 0.66 (95% confidence interval (CI) 0.59‒0.73) at year 1, and 0.41 (95% CI 0.34‒0.48) at year 4. That is, of 100 patients on etanercept therapy at baseline, on average 66 remained on etanercept therapy 1 year later, and 41 remained on the same therapy 4 years later. The drug survival rate dropped from 0.69 (95% CI 0.62‒0.75) at year 1 to 0.47 (95% CI 0.42‒0.52) at year 4 for adalimumab, from 0.61 (95% CI 0.54‒0.67) to 0.42 (95% CI 0.34‒0.50) for infliximab, and from 0.82 (95% CI 0.79‒0.86) to 0.56 (95% CI 0.42‒0.70) for ustekinumab. Overall, etanercept was associated with the lowest drug survival rate and ustekinumab associated with the highest one.
Figure 2.
Drug survival of biologics for treating psoriasis in (a) all subjects, (b) biologic-naïve subjects, and as to (c) discontinue due to loss of efficacy and (d) adverse effects.
Subgroup analysis on biologic-naïve subjects
The subgroup analysis limited to biologic-naïve subjects is illustrated in Fig. 2(b), with the forest plots shown in Supplementary Fig. S2. There were 7 studies (2,536 patients) reporting data on biologic-naïve patients for etanercept, 7 studies (2,455 patients) for adalimumab, 4 studies (136 patients) for infliximab, and 4 studies (368 patients) for ustekinumab. The drug survival in biologic-naïve patients was generally higher than that in all subjects. The drug survival in biologic-naïve subjects gradually decreased with time for all biologics, too. The drug survival rate fell from 0.67 (95% CI 0.53‒0.80) at year 1 to 0.52 (95% CI 0.41‒0.64) at year 4 for etanercept, from 0.67 (95% CI 0.49‒0.86) to 0.63 (95% CI 0.45‒0.82) for adalimumab, from 0.57 (95% CI 0.19‒0.95) to 0.47 (95% CI 0.16‒0.79) for infliximab, and from 0.88 (95% CI 0.77‒0.99) to 0.87 (95% CI 0.87‒0.87) for ustekinumab. Ustekinumab had a trough drug survival at year 3 because the study of Mitratza et al.26. Contributed data to the low survival at year 3. In general, ustekinumab was associated with the highest drug survival rate and infliximab associated with the lowest rate in biologic-naïve subjects.
Discontinuation of biologics due to loss of efficacy
The data on discontinuation due to loss of efficacy are shown in Table 1. There were 5 studies (2,338 patients) reporting data for etanercept, 6 studies (3,580 patients) for adalimumab, 3 studies (368patients) for infliximab, and 5 studies (1,249 patients) for ustekinumab. The meta-analysis on drug survival as to discontinuation due to loss of efficacy from year 1 to 4 is shown as Fig. 2(c) and forest plot is show in Supplementary Fig. S3. The drug survival rate fell from 0.80 (95% CI 0.80‒0.81) at year 1 to 0.47 (95% CI 0.42‒0.53) at year 4 for etanercept, from 0.88 (95% CI 0.84‒0.92) to 0.57 (95% CI 0.52‒0.53) for adalimumab, from 0.87 (95% CI 0.77‒0.97) to 0.51 (95% CI 0.47‒0.56) for infliximab, and from 0.93 (95% CI 0.90‒0.99) to 0.79 (95% CI 0.72‒0.87) for ustekinumab. Etanercept was the biologics most commonly associated with discontinuation due to loss of efficacy and ustekinumab was the least one.
Table 1.
Studies reporting discontinuation of biologics due to loss of efficacy or adverse effects.
| References | Patient papulation | Drug survival rate | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 10 years | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Loss of efficacy | |||||||||||||||
| Etanercept | |||||||||||||||
| Zweegers et al.36 | 238 | 193 | 81.1 | 161 | 67.6 | 148 | 62.2 | 122 | 51.4 | 107 | 45 | — | — | 74 | 31 |
| Vilarrasa et al.34 | 248 | 192 | 77.6 | 142 | 57.1 | 116 | 46.9 | 101 | 40.8 | — | — | — | — | — | — |
| Warren et al.35 | 1098 | 878 | 80 | 714 | 65 | 604 | 55 | — | — | — | — | — | — | — | — |
| Van den Reek et al.31 | 82 | 68 | 83 | — | — | — | — | — | — | — | — | — | — | — | — |
| Davila-Seijo et al.13 | 672 | 543 | 80.8 | 439 | 65.4 | 362 | 53.9 | 336 | 50.0 | ||||||
| Adalimumab | |||||||||||||||
| Zweegers et al.36 | 186 | 161 | 86.5 | 136 | 73.0 | 121 | 64.9 | 116 | 62.2 | 100 | 54 | 93 | 50 | ||
| Vilarrasa et al.34 | 231 | 188 | 81.6 | 155 | 67.3 | 141 | 61.2 | 118 | 51 | — | — | — | — | — | — |
| Van den Reek et al.41 | 459 | — | — | — | — | — | — | 285 | 62 | — | — | — | — | — | — |
| Warren et al.35 | 1879 | 1691 | 90 | 1560 | 83 | 1484 | 79 | — | — | — | — | — | — | — | — |
| Van den Reek et al.31 | 101 | 86 | 85 | — | — | — | — | — | — | — | — | — | — | — | — |
| Davila-Seijo et al.13 | 724 | 696 | 96.2 | 487 | 67.3 | 446 | 61.5 | 390 | 53.9 | ||||||
| Infliximab | |||||||||||||||
| Vilarrasa et al.34 | 84 | 67 | 79.6 | 55 | 65.3 | 48 | 57.1 | 41 | 49 | — | — | — | — | — | — |
| Warren et al.35 | 96 | 83 | 86 | 76 | 79 | 73 | 76 | — | — | — | — | — | — | — | — |
| Davila-Seijo et al.13 | 188 | 181 | 96.2 | 130 | 69.2 | 108 | 57.7 | 101 | 53.9 | ||||||
| Ustekinumab | |||||||||||||||
| Zweegers et al.36 | 102 | 94 | 91.9 | 88 | 86.5 | 88 | 86.5 | 85 | 83.8 | 81 | 79 | — | — | — | — |
| Vilarrasa et al.34 | 140 | 131 | 93.8 | 131 | 93.8 | 120 | 85.7 | 114 | 81.6 | — | — | — | — | — | — |
| Warren et al.35 | 450 | 432 | 96 | 419 | 93 | 401 | 89 | — | — | — | — | — | — | — | — |
| Van den Reek et al.31 | 66 | 62 | 94 | — | — | — | — | — | — | — | — | — | — | — | — |
| Davila-Seijo et al.13 | 491 | 444 | 90.4 | 415 | 84.6 | 378 | 76.9 | 359 | 73.1 | ||||||
| Adverse effects | |||||||||||||||
| Etanercept | |||||||||||||||
| Zweegers et al.36 | 238 | 228 | 95.9 | 212 | 89.2 | 212 | 89.2 | 212 | 89.2 | — | — | — | — | 167 | 70 |
| Warren et al.35 | 1098 | 1032 | 94.0 | 999 | 91.0 | 988 | 90.0 | — | — | — | — | — | — | — | — |
| Van den Reek et al.31 | 82 | 74 | 90.0 | — | — | — | — | — | — | — | — | — | — | — | — |
| Davila-Seijo et al.13 | 672 | 620 | 92.3 | 594 | 88.5 | 569 | 84.6 | 597 | 88.8 | ||||||
| Adalimumab | |||||||||||||||
| Zweegers et al.36 | 186 | 166 | 89.2 | 156 | 83.8 | 153 | 82.4 | 151 | 81.1 | — | — | 130 | 70 | — | — |
| Van den Reek41 | 459 | — | — | — | — | — | — | 386 | 84.0 | — | — | — | — | — | — |
| Warren et al.35 | 1879 | 1747 | 93.0 | 1681 | 90.0 | 1635 | 87 | — | — | — | — | — | — | — | — |
| Van den Reek et al.31 | 101 | 96 | 95.0 | — | — | — | — | — | — | — | — | — | — | — | — |
| Davila-Seijo et al.13 | 724 | 668 | 92.3 | 640 | 88.5 | 640 | 88.5 | 613 | 84.6 | ||||||
| Infliximab | |||||||||||||||
| Warren et al.35 | 96 | 81 | 84.0 | 71 | 74.0 | 57 | 59.0 | — | — | — | — | — | — | — | — |
| Davila-Seijo et al.13 | 188 | 159 | 84.6 | 152 | 80.8 | 145 | 76.9 | 137 | 73.1 | ||||||
| Ustekinumab | |||||||||||||||
| Zweegers et al.36 | 102 | 94 | 91.9 | 85 | 83.8 | 85 | 83.8 | 77 | 75.7 | ||||||
| Warren et al.35 | 450 | 432 | 96.0 | 419 | 93.0 | 410 | 91.0 | — | — | — | — | — | — | — | — |
| Van den Reek et al.31 | 66 | 63 | 95.0 | — | — | — | — | — | — | — | — | — | — | — | — |
| Davila-Seijo et al.13 | 491 | 491 | 100.0 | 472 | 96.2 | 472 | 96.2 | 472 | 96.2 | ||||||
Discontinuation of biologics due to adverse effects
The data on discontinuation due to adverse effects are reported in Table 1. There were 4 studies (2,090 patients) providing data for etanercept, 5 studies (3,349 patients) for adalimumab, 2 studies (284 patients) for infliximab, and 4 studies (1,109 patients) for ustekinumab. The meta-analysis on the drug survival as to discontinuation due to adverse effects is illustrated in Fig. 2(d) and the forest plots are shown in Supplementary Fig. S4. Ustekinumab was the least frequently associated with discontinuation due to adverse effects. On the other hand, infliximab was most frequently associated with discontinuation due to adverse effects.
Discussion
Main findings
To the best of our knowledge, this study is the first to conduct a meta-analysis on the drug survival of biologics for treating psoriasis. We found ustekinumab had the best drug survival in all subjects (including biologic-naïve and experienced ones) and had significantly higher drug survival than the other biologics in the first three years. Ustekinumab had the highest drug survival in biologic-naïve subjects, but did not have a significantly higher drug survival than the other biologics. Etanercept had the worst drug survival in the primary analysis on all subjects; but when limited to biologic-naïve patients, infliximab had the worst drug survival. Etanercept was most often associated with discontinuation due to loss of efficacy, while ustekinumab was the least common. Ustekinumab were the least likely to be discontinued due to adverse effects. Infliximab were the most common biologics discontinued due to adverse effects.
Strengths and limitations
Our study has several strengths. Firstly, this study included 37 studies with 32,231 subjects, and the average number of study subjects for each biologic was 8,158 patients. Thus, a large sample was available for this meta-analysis on the drug survival. Secondly, the available data enabled us to conduct subgroup analysis on biologic-naïve subjects, discontinuation due to loss of efficacy and adverse effects. Therefore, our study provides drug survival data for various clinical scenarios in the real world. Thirdly, we did not impose any language limitations and thus increased the scope of data searched and collected.
On the other hand, our study had some limitations. Firstly, the study population of included studies were mainly from Europe and thus contained very few Asian patients. Secondly, those studies were observational and might have biases to influence the drug survival, such as selection bias due to non-random allocation. However, randomized trials on drug survival are unlikely to be conducted by pharmaceutical companies. Thirdly, other patients’ baseline characteristics, such as patients’ body weight, age, gender, comorbidities, and co-treatments for psoriasis, might have influenced the drug survival which was reflected by high statistical heterogeneity. However, we could not adjust these factors in our analysis because the included studies did not have relevant details, except for one study36 showing a high body mass index and female sex might have affected the drug survival. Fourthly, drug survival could be a surrogate of treatment efficacy and safety to aid clinical making decision. Although we performed subgroup analyses on discontinuation due to loss of efficacy and adverse effects which may be a useful reference for clinical practice, the available sample size was limited. Fifthly, we did not have adequate data on drug survival of biologics newly available on the market, for example secukinumab and ixekizumab. Sixthly, due to the lack of information on costs and insurance plan for each study subjects, we could not examine their effects on drug survival. For example, people in the US can change their insurance plan. However, there was only one US study included in our analysis14.
Implications for practice
The current evidence suggests that ustekinumab is associated with the best drug survival in all and biologic-naïve subjects. Also, ustekinumab is the biologic least frequently associated with discontinuation due to loss of efficacy. Adalimumab has the second-best drug survival. Etanercept has the worst overall drug survival and is the biologic most commonly discontinued due to loss of efficacy. Infliximab has the lowest drug survival among biologic-naïve subjects.
No et al.6. conducted a systematic review including 21 studies with 20,297 subjects, which was smaller than our study. They reported the median drug survival for ustekinumab, infliximab, adalimumab and etanercept were 38.0 months, 36.5 months, 26.6 months, and 24.7 months, separately. Although they claimed ustekinumab had the highest drug survival, there were no significant differences between ustekinumab and other biologics as the interquartile range of their median drug survival overlapped. They found the drug survival gradually decreased and ustekinumab had the best drug survival, which were in congruent with our study. The study of No et al.6 only included studies published in English with at least 50 subjects aged at least 18 years; by contrast, our study had no limitations in language, participant characteristics, and sample size. The difference in the inclusion and exclusion criteria of the two studies might have accounted for the discrepancy in the biologic with the second-best drug survival.
The included drug survival studies did not consider the costs of treatments. One previous study on the cost-efficacy of biologics for treating psoriasis demonstrates ustekinumab and adalimumab had the lowest 1-year cost per PASI 75 responder, while ustekinumab had the lowest 2-year cost per PASI 75 responder46. Clinicians may consider the data on drug survival and cost efficacy in choosing biologics for treating psoriasis to achieve clinical benefit at the least medical expenditure.
Conclusions
Drug survival represents the long-term effectiveness and safety in the real-world setting, and is a useful reference for clinical practice. The current evidence suggests ustekinumab is the preferred biologics for treating psoriasis. Further studies are required to complement the current limited data on discontinuation due to loss of efficacy and adverse effects. Clinicians may consider the data on drug survival in choosing biologics in treating psoriasis.
Methods
Identification of studies and data extraction
We searched the PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and EMBASE databases from inception to 7th October 2017 for relevant studies. To increase the sensitivity of search, we also used snowball method, i.e. we scanned the references list of included studies to see if there were other relevant studies. We used the following terms in searching electronic databases: “drug retention” or “drug survival” or “drug persistence” and “psoriasis”. Studies that analyzed the drug survival of biologics and reported the respective annual data for each biologic for at least 1 year were included. Studies that did not report the respective survival of biologics or did not have outcome data for at least 1 year were excluded.
We scanned the titles and abstracts of the search results and obtained the full text of potentially eligible studies for further evaluation. Two investigators (PL and CC) independently screened, selected relevant studies, and used the NOS8 to assess the risk of bias of included studies. A third author (SW) was available for arbitration when discrepancy occurred. We did not impose any language restrictions.
Risk of bias assessment
The NOS uses three categories (selection of study groups, comparability, and outcome assessment) to evaluate the risk of bias of cohort studies. As to the selection category, four items are evaluated, including (1) the representativeness of exposed cohort, (2) selection of non-exposed cohort, (3) ascertainment of exposure, and (4) outcome of the interest not present at start of study. Regarding the comparability category, the comparability of cohorts was assessed. In the outcome category, two items were rated: (1) follow-up duration and (2) adequacy of follow up of cohorts. A study could be awarded up to one star for each numbered item within the selection and outcome categories and up to two stars for comparability. The highest quality studies are awarded up to nine stars8.
Data extraction
We used a pre-determined data extraction form to collect data including the first author’s last name, publication year, type of biologics, a history of receiving any biologics before or not (biologic-naïve or -experienced status), sample size, survival (percentage or number of patients) per year (from year 1 to 4), and the respective rate of withdrawal due to loss of efficacy and adverse effects. We did not extract data of efalizumab which has been withdrawn from the market.
Statistical analysis
The drug survival rate and numbers of patients of each biologic in each study were collected. As we expected clinical heterogeneity across the included studies, we implemented a random-effects model meta-analysis using the generic inverse variance method to calculate the pooled drug survival rate of each biologic. The drug survival rate was expressed as mean difference (MD) with 95% confidence interval (CI), which was 1 at baseline and represented 100% of patients received the biologic therapy. When the MD dropped to for example 0.50 in following years, 50% of patients remained on the same biologic therapy. When the MD was 0, all the patients discontinued the biologic therapy.
We further conducted a subgroup analysis limited to biologic-naïve subjects. The drug survival as to discontinuation due to loss of efficacy and adverse effects was analyzed separately. The Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for conducting meta-analysis.
Electronic supplementary material
Author Contributions
This study was designed by C.C. Data extraction was performed by P.L. and C.C. Data review was done by all authors. First draft was written by P.L. All authors commented on the first draft and agreed with the final version. C.C. is the guarantor of the study.
Competing Interests
Ms Lin and Dr Wang have no conflicts to report; Prof Chi received speaking fees from AbbVie Taiwan, Janssen-Cilag Taiwan, Novartis Taiwan, and Pfizer Taiwan.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34293-y.
References
- 1.Chi CC, Chen TH, Wang SH, Tung TH. Risk of suicidality in people with psoriasis: a systematic review and meta-analysis of cohort studies. Am J Clin Dermatol. 2017;18:621–627. doi: 10.1007/s40257-017-0281-1. [DOI] [PubMed] [Google Scholar]
- 2.Chi Ching-Chi, Tung Tao-Hsin, Wang Jui, Lin Yu-Sheng, Chen Yu-Fen, Hsu Tsui-Kan, Wang Shu-Hui. Risk of Uveitis Among People With Psoriasis. JAMA Ophthalmology. 2017;135(5):415. doi: 10.1001/jamaophthalmol.2017.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai TF, et al. Taiwanese Dermatological Association consensus statement on management of psoriasis. Dermatologica Sinica. 2017;35:66–77. doi: 10.1016/j.dsi.2017.01.002. [DOI] [Google Scholar]
- 4.Wang SH, Chi CC, Hu S. Cost‐efficacy of biologic therapies for moderate to severe psoriasis from the perspective of the Taiwanese healthcare system. Int J Dermatol. 2014;53:1151–1156. doi: 10.1111/ijd.12462. [DOI] [PubMed] [Google Scholar]
- 5. Sbidian, E. et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis (Review) Cochrane Database Syst Rev, CD011535, 10.1002/14651858 (2017). [DOI] [PMC free article] [PubMed]
- 6.No Daniel J., Inkeles Megan S., Amin Mina, Wu Jashin J. Drug survival of biologic treatments in psoriasis: a systematic review. Journal of Dermatological Treatment. 2017;29(5):460–466. doi: 10.1080/09546634.2017.1398393. [DOI] [PubMed] [Google Scholar]
- 7.Moher DLA, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells, G. et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 9.Arnold T, et al. Drug survival rates and reasons for drug discontinuation in psoriasis. J Dtsch Dermatol Ges. 2016;14:1089–1099. doi: 10.1111/ddg.13152. [DOI] [PubMed] [Google Scholar]
- 10.Bonafede M, Johnson BH, Fox KM, Watson C, Gandra SR. Treatment patterns with etanercept and adalimumab for psoriatic diseases in a real-world setting. J Dermatolog Treat. 2013;24:369–373. doi: 10.3109/09546634.2012.755255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunasso AM, Puntoni M, Massone C. Drug survival rates of biologic treatments in patients with psoriasis vulgaris. Br J Dermatol. 2012;166:447–449. doi: 10.1111/j.1365-2133.2011.10557.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan B, et al. One-year drug retention in individuals enrolled in an etanercept patient support program. J Rheumatol. 2010;37:1323. doi: 10.3899/jrheum.090168. [DOI] [Google Scholar]
- 13.Davila-Seijo P, et al. Survival of classic and biological systemic drugs in psoriasis: results of the Biobadaderm registry and critical analysis. J Eur Acad Dermatol Venereol. 2016;30:1942–1950. doi: 10.1111/jdv.13682. [DOI] [PubMed] [Google Scholar]
- 14.Doshi JA, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol. 2016;74:1057–1065.e1054. doi: 10.1016/j.jaad.2016.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito M, et al. Survival rate of antitumour necrosis factor-α treatments for psoriasis in routine dermatological practice: a multicentre observational study. Br J Dermatol. 2013;169:666–672. doi: 10.1111/bjd.12422. [DOI] [PubMed] [Google Scholar]
- 16.Gniadecki R, et al. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172:244–252. doi: 10.1111/bjd.13343. [DOI] [PubMed] [Google Scholar]
- 17.Gniadecki R, Kragballe K, Dam TN, Skov L. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol. 2011;164:1091–1096. doi: 10.1111/j.1365-2133.2011.10213.x. [DOI] [PubMed] [Google Scholar]
- 18.Inzinger M, et al. Survival and effectiveness of tumour necrosis factor-alpha inhibitors in the treatment of plaque psoriasis under daily life conditions: Report from the psoriasis registry Austria. Acta Derm Venereol. 2016;96:207–212. doi: 10.2340/00015555-2214. [DOI] [PubMed] [Google Scholar]
- 19.Jacobi A, Rustenbach SJ, Augustin M. Comorbidity as a predictor for drug survival of biologic therapy in patients with psoriasis. Int J Dermatol. 2016;55:296–302. doi: 10.1111/ijd.12879. [DOI] [PubMed] [Google Scholar]
- 20.Kimball AB, et al. OBSERVE-5: observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72:115–122. doi: 10.1016/j.jaad.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Lernia V, Tasin L, Pellicano R, Zumiani G, Albertini G. Impact of body mass index on retention rates of anti-TNF-alfa drugs in daily practice for psoriasis. J Dermatolog Treat. 2012;23:404–409. doi: 10.3109/09546634.2011.593489. [DOI] [PubMed] [Google Scholar]
- 22.Marinas JE, et al. Survival rates of biological therapies for psoriasis treatment in real-world clinical practice: A Canadian multicentre retrospective study. Australas J Dermatol. 2018;59:e11–e14. doi: 10.1111/ajd.12548. [DOI] [PubMed] [Google Scholar]
- 23.Menter A, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Eur Acad Dermatol Venereol. 2016;30:1148–1158. doi: 10.1111/jdv.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menting SP, et al. Drug survival is not significantly different between biologics in patients with psoriasis vulgaris: a single-centre database analysis. Br J Dermatol. 2014;171:875–883. doi: 10.1111/bjd.13001. [DOI] [PubMed] [Google Scholar]
- 25.Mercadal Orfila G, et al. Persistence and cost of biologic agents for psoriasis: Retrospective study in the Balearic Islands. Eur J Clin Pharm. 2016;18:163–170. [Google Scholar]
- 26.Mitratza M, et al. Drug survival in psoriatic patients treated with biologic agent for the first time. J Am AcadDermatol. 2017;76:AB106. doi: 10.1016/j.jaad.2017.04.423. [DOI] [Google Scholar]
- 27.Pogácsás L, et al. Long-term drug survival and predictor analysis of the whole psoriatic patient population on biological therapy in Hungary. J Dermatolog Treat. 2017;28:635–647. doi: 10.1080/09546634.2017.1329504. [DOI] [PubMed] [Google Scholar]
- 28.Richter L, et al. Etanercept, adalimumab, and ustekinumab in psoriasis: analysis of 209 treatment series in Austria. J Dtsch Dermatol Ges. 2017;15:309–317. doi: 10.1111/ddg.13191. [DOI] [PubMed] [Google Scholar]
- 29.Ross C, Marshman G, Grillo M, Stanford T. Biological therapies for psoriasis: Adherence and outcome analysis from a clinical perspective. Australas J Dermatol. 2016;57:137–140. doi: 10.1111/ajd.12294. [DOI] [PubMed] [Google Scholar]
- 30.Shalom G, et al. Biologic drug survival in Israeli psoriasis patients. J Am Acad Dermatol. 2016;76:662–669. doi: 10.1016/j.jaad.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Van den Reek JM, et al. ‘Happy’ drug survival of adalimumab, etanercept and ustekinumab in psoriasis in daily practice care: results from the BioCAPTURE network. Br J Dermatol. 2014;171:1189–1196. doi: 10.1111/bjd.13087. [DOI] [PubMed] [Google Scholar]
- 32.Van den Reek JM, et al. Determinants of drug survival for etanercept in a long-term daily practice cohort of patients with psoriasis. Br J Dermatol. 2014;170:415–424. doi: 10.1111/bjd.12648. [DOI] [PubMed] [Google Scholar]
- 33.Ventayol P. Drug survival rates and cost of biological agents for the treatment of moderate to severe psoriasis in the Balearic Islands (Spain) Value Health. 2014;17:A610. doi: 10.1016/j.jval.2014.08.2135. [DOI] [PubMed] [Google Scholar]
- 34.Vilarrasa E, et al. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): A retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatology. 2016;74:1066–1072. doi: 10.1016/j.jaad.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 35.Warren RB, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: A prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2015;135:2632–2640. doi: 10.1038/jid.2015.208. [DOI] [PubMed] [Google Scholar]
- 36.Zweegers J, et al. Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side-effects in psoriasis patients treated with adalimumab, etanercept or ustekinumab in daily practice. A prospective, comparative, long-term drug survival study from the BioCAPTURE registry. Br J Dermatol. 2016;175:340–347. doi: 10.1111/bjd.14552. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Ferrer A, Vilarrasa E, Gich IJ, Puig L. Adalimumab for the treatment of psoriasis in real life: a retrospective cohort of 119 patients at a single Spanish centre. Br J Dermatol. 2013;169:1141–1147. doi: 10.1111/bjd.12543. [DOI] [PubMed] [Google Scholar]
- 38.Spertino J, Lopez-Ferrer A, Vilarrasa E, Puig L. Long-term study of infliximab for psoriasis in daily practice: drug survival depends on combined treatment, obesity and infusion reactions. J Eur Acad Dermatol Venereol. 2014;28:1514–1521. doi: 10.1111/jdv.12331. [DOI] [PubMed] [Google Scholar]
- 39.Suarez-Perez JA, Herrera E, Acosta EH, Fernández MVM, Cuervas PM. Efficacy and drug survival rates for adalimumab in patients with psoriasis vulgaris. J Am Acad Dermatol. 2014;70:AB168. [Google Scholar]
- 40.Umezawa Y, et al. Drug survival rates in patients with psoriasis after treatment with biologics. J Dermatol. 2013;40:1008–1013. doi: 10.1111/1346-8138.12353. [DOI] [PubMed] [Google Scholar]
- 41.Van den Reek JM, et al. Adalimumab drug survival in patients with psoriasis, Crohn's disease, and rheumatoid arthritis: Relevant differences using the same treatment. J Am Acad Dermatol. 2016;74:177–179. doi: 10.1016/j.jaad.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Van den Reek JM, et al. Predictors of adalimumab drug survival in psoriasis differ by reason for discontinuation: long-term results from the Bio-CAPTURE registry. J Eur Acad Dermatol Venereol. 2015;29:560–565. doi: 10.1111/jdv.12636. [DOI] [PubMed] [Google Scholar]
- 43.Van Den Reek JM, et al. Differences between adalimumab drug survival rates in Crohn disease, rheumatoid arthritis and psoriasis. Br J Dermatol. 2014;171:e131–e132. doi: 10.1111/bjd.12998. [DOI] [Google Scholar]
- 44.García-Martínez P, Gallardo F, Gimeno R, Pujol RM, Ferran M. Experience with ustekinumab for the treatment of moderate-to-severe cutaneous psoriasis in our clinical practice setting. J Invest Dermatol. 2015;135:S19. doi: 10.1038/jid.2014.444. [DOI] [Google Scholar]
- 45.Suarez-Perez JA, et al. Survival rate of ustekinumab for psoriasis in real life: A multicenter observational study. J Am Acad Dermatol. 2015;72:AB253. [Google Scholar]
- 46.Chi Ching-Chi, Wang Shu-Hui. Efficacy and Cost-Efficacy of Biologic Therapies for Moderate to Severe Psoriasis: A Meta-Analysis and Cost-Efficacy Analysis Using the Intention-to-Treat Principle. BioMed Research International. 2014;2014:1–10. doi: 10.1155/2014/862851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.