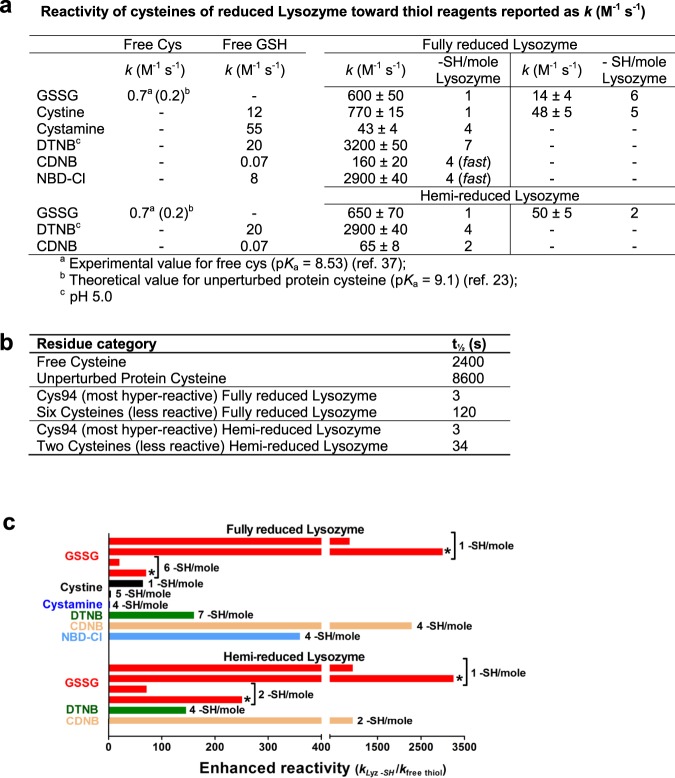
Figure 2.
Reactivity of Lyzred toward different disulfides and thiol reagents. (a) Second order kinetic constants k (M−1 s−1) for the reaction of the cysteines of Lyzred and hemi-reduced Lyz, free Cys and free GSH with natural disulfides and other thiol reagents calculated at pH 7.4 and 25 °C (DTNB at pH 5.0). Errors are reported as S.D. from five independent experiments. (b) t1/2 for the reaction of cysteines of Lyzred and hemi-reduced Lyz with GSSG at pH 7.4 and 25 °C. (c) Second order kinetic constants of Lyzred and hemi-reduced Lyz toward GSSG normalized to the corresponding constants found for free Cys (0.7 M−1 s−1) or () calculated for unperturbed protein Cys (0.2 M−1 s−1) (see Methods section). All other bars represent the second order kinetic constants of Lyzred and hemi-reduced Lyz in its reactions with other disulfides or thiol reagents normalized to the corresponding constants calculated for GSH. Note that electrostatic factors may have a critical role in determining the different kinetic constants for the reaction of free GSH (negatively charged) with cystine (neutral) and cystamine (positively charged).