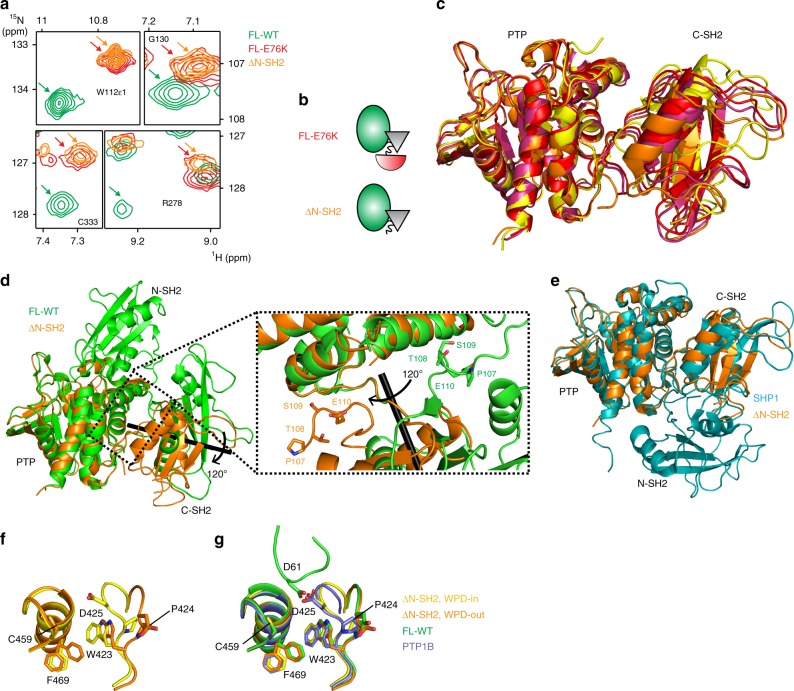
Fig. 2.
Structure of the open active state of SHP2. a Chemical shifts of ΔN-SH2 are nearly identical to FL-E76K indicating that removal of the N-SH2 mimics the structure of FL-E76K, shown as cartoon in b. c The four molecules found in the asymmetric unit of the ΔN-SH2 crystal structure (purple, red, orange, yellow) were superimposed using the PTP domain as reference to reveal an ensemble of orientations adopted by the C-SH2 domain. d When compared with FL-WT (green), the C-SH2 domain of the ΔN-SH2 structure (orange) is rotated by 120° (axis in black). This movement positions the SH2-SH2 linker in between the PTP domain and the C-SH2 as exemplified by residues P107-E110 (inset). e The C-SH2 domain in the ΔN-SH2 open structure (orange) adopts a similar conformation to the C-SH2 in the open state of SHP1 (PDB 3ps5, teal)21. f Zoom-in of the active site of ΔN-SH2: conformational sampling of the WPD-in (yellow) and WPD-out states (orange, seen in all previous SHP2 structures). g The N-SH2 loop from FL-WT (green) can only dock into the PTP domain when the WPD is in the out conformation. The WPD-in conformation of ΔN-SH2 crystal structure is identical to the one reported for the PTP1B (PDB 1sug, blue)24