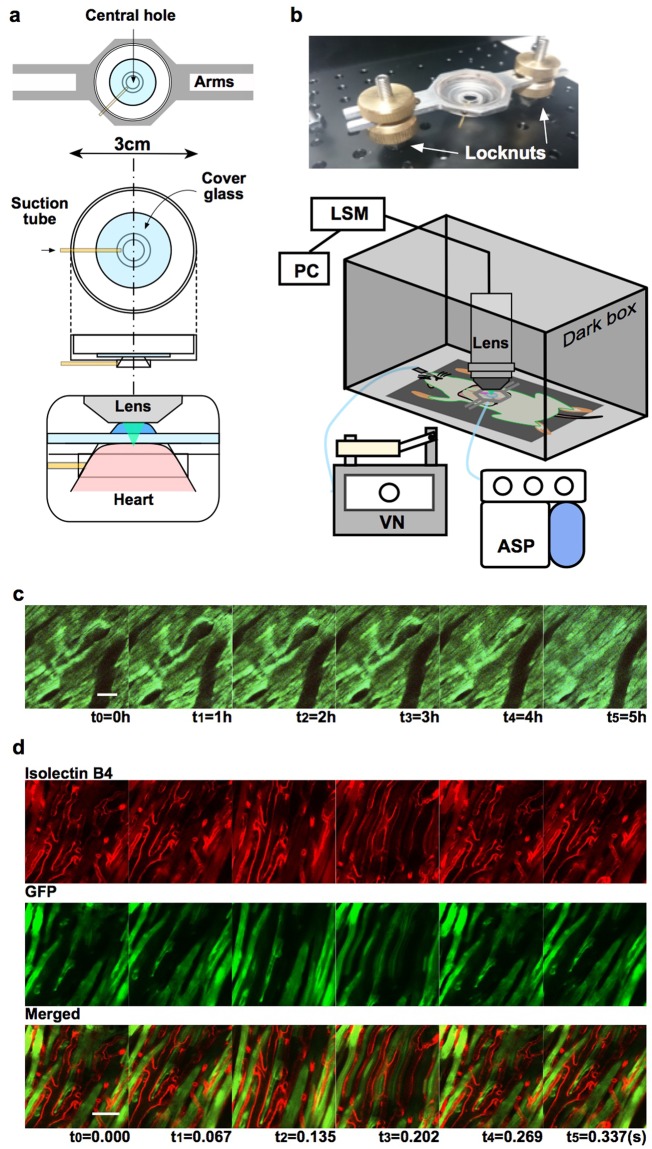
Figure 1.
Experimental setup and real-time in vivo imaging of normal heart beating. (a) Stabiliser design: a central hole with a diameter of 8 mm diameter and a depth of 1 mm was cut and then completely countersunk at a 50° angle to the plane of the stabiliser. The hole was covered with a round coverglass. A suction tube could access the chamber through the lateral wall of the central hole—which protruded slightly above the plane of the stabiliser—and could attach to the heart surface by suction, as shown in the enlarged side view. (b) After optimising the height from the steel plate, the stabiliser was fixed by interpolating the bilateral arms between locknuts on two threaded pillars protruding from the plate. The equipment included a water-dipping objective lens, ventilator (VN), aspirator (ASP), laser-scanning microscope (LSM), and personal computer (PC). (c) Longitudinal time-lapse imaging of the beating heart in a GFP rat. The cardiomyocyte structure was labelled with GFP. No photobleaching occurred during recording at 15 frames/s (Supplementary Fig. 1). Scale bar, 100 µm. Panels (t0–t5) show representative frames recorded every hour. (d) Visualisation of the dynamic motion of capillaries between cardiac myocytes. The endothelium of the microvasculature was stained with isolectin B4 (red, left panels); cardiomyocytes were labelled with GFP (green, middle panels). Scale bar, 50 µm (Supplementary Video 1). Panels (t0–t5) show representative frames recorded consecutively over a single cardiac cycle (15 frames/s).