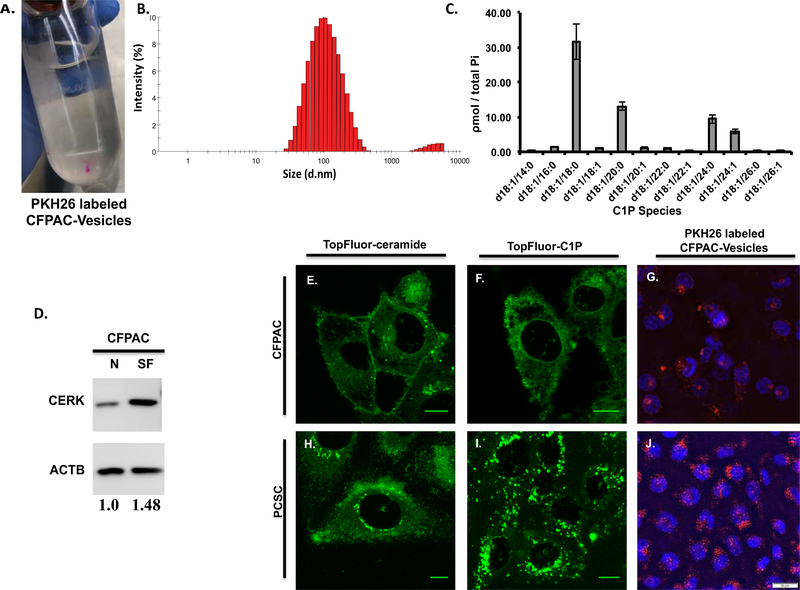
Figure 6: Extracellular vesicles secreted in CFPAC-conditioned medium are rich in C1P and C1P uptake is peri-nuclear in progenitor cells and is similar to uptake of CFPAC secreted C1P-containing extracellular vesicles.
The size of vesicles isolated using a sucrose gradient from CFPAC conditioned medium were assessed using Dynamic Light Scattering (DLS) where 95.88% were found to be 116nm ± 3.5 SEM in size while the remaining 4.125% were 4219nm ± 11.7 SEM (B) (n=4 unique preparations, 3 runs per preparation and a minimum of 12 measurements per run for a total of 144 measurements). Secreted vesicle isolates from serum-free (SF) CFPAC cells were noted to contain C1P species consistent with that seen in whole cell lysate (normalized to total phosphate: Pi, nmole/sample) (C). CFPAC cells in serum-free (SF) medium by densitometry analysis have a 1.48 fold increase in CERK compared to cells in normal medium (N) (D, n=2, representative blot). TopFluor labeled ceramide and C1P lipid uptake in sub-confluent PCSC and CFPAC cells. PCSCs exhibit a marked increase in C1P uptake in the perinuclear region (I) as compared to CFPAC (F) or TopFluor-ceramide (H,E). Conditioned medium from CFPAC cells was collected; extracellular vesicles isolated and surfaced labeled (A, PKH26 red fluorescent dye) and added to subconfluent cells (n=3 on different occasions). Perinuclear uptake of extracellular vesicles was significantly greater in PCSCs (J) as compared to CFPAC cells (G) (G,J: DAPI staining denotes nucleus) (n=3 on different occasions). Scale bar: 10μm(E,F,H,I), 20μm(G,J).