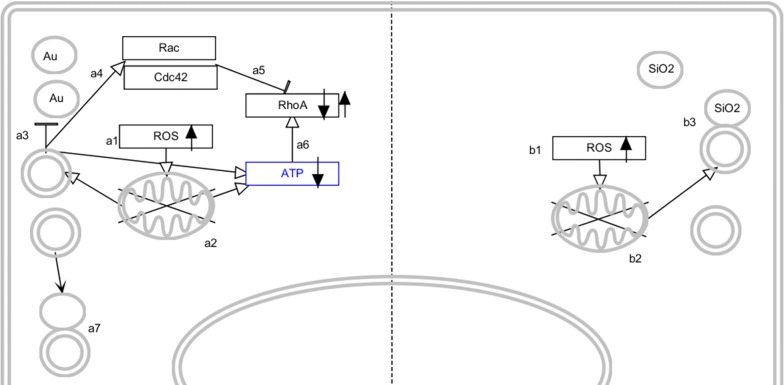
Scheme 1.
Schematic representation of the cellular effects of Au (left side) and SiO2 NP (right side) exposure. Au NPs enter the cells and induce ROS (a1) which causes mitochondrial damage (a2). This stimulates autophagy induction, but autophagosomes cannot be efficiently cleared due to the reduced lysosomal activity (a3), resulting in an accumulation of autophagosomes. This stimulates Rac and Cdc42 activity (a4), which affects cytoskeletal organization and actin-mediated signaling. Simultaneously, Cdc42 and Rac inhibit RhoA activity (a5). Mitochondrial damage and accumulation of autophagosomes lowers cellular ATP levels which in turn stimulates RhoA activity. Upon recovery of the cellular degradative capacity, autophagosome clearance occurs more efficiently (a7), which reduces cellular autophagy levels and Rac and Cdc42 activity. The low ATP levels then result in increased RhoA activity, which will return back to baseline levels as turnover of damaged mitochondria occurs more efficiently. RhoA activity will restore the cellular cytoskeleton and actin-mediated signaling. SiO2 NPs also enter the cells and induce ROS (b1), which affects mitochondrial health. This stimulates autophagy (b2) which promotes clearance of the damaged mitochondria. Upon fusion with the lysosomes, mitochondrial turnover remains normal, resulting in an efficient management of cellular ATP levels