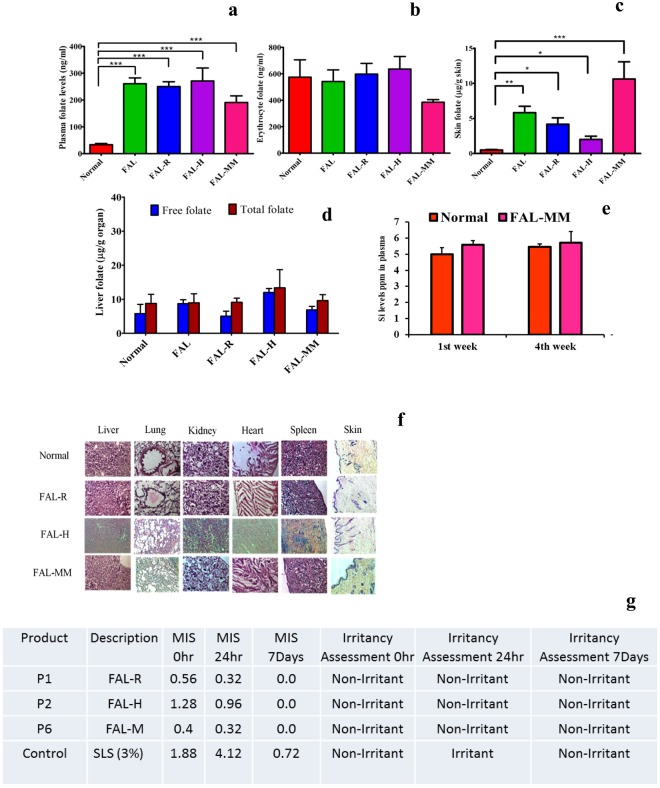
Figure 5.
Sub-acute repeat dose and skin safety in rats (5 mg/kg FA dose/daily for 4 weeks) and Dermatological safety evaluation in 25 humans (Single application of RDA dose 400 µg/person). (a) Plasma profile in normal rats with significant folate levels due to Nutricosmetics. (b) Erythrocyte folate levels. (c) Skin folate levels showing reservoir of FA after 4 weeks. (d) Liver folate levels shows no toxicity in liver. (e) No significant Silicon levels(ppm) in plasma after 28 days daily FAL-MM application proves safety of FAL-MM. (f) Histopathology of H&E stained organs of rats sacrificed after 4 weeks observed under 10X. Normal group given regular diet throughout study whereas other groups were given folate deficient diet though out the study duration (a–f) N = 6 Mean ± SD ***P < 0.001, **P < 0.05 and *P < 0.01, Student’s t-test. (g) Skin compatibility dermatological evaluated of Nutricosmetics in humans shows no irritation potential for topical application in human volunteers.