Fig. 7. Model for SFK-Cbl axis-dependent negative regulation of the NLRP3 inflammasome.
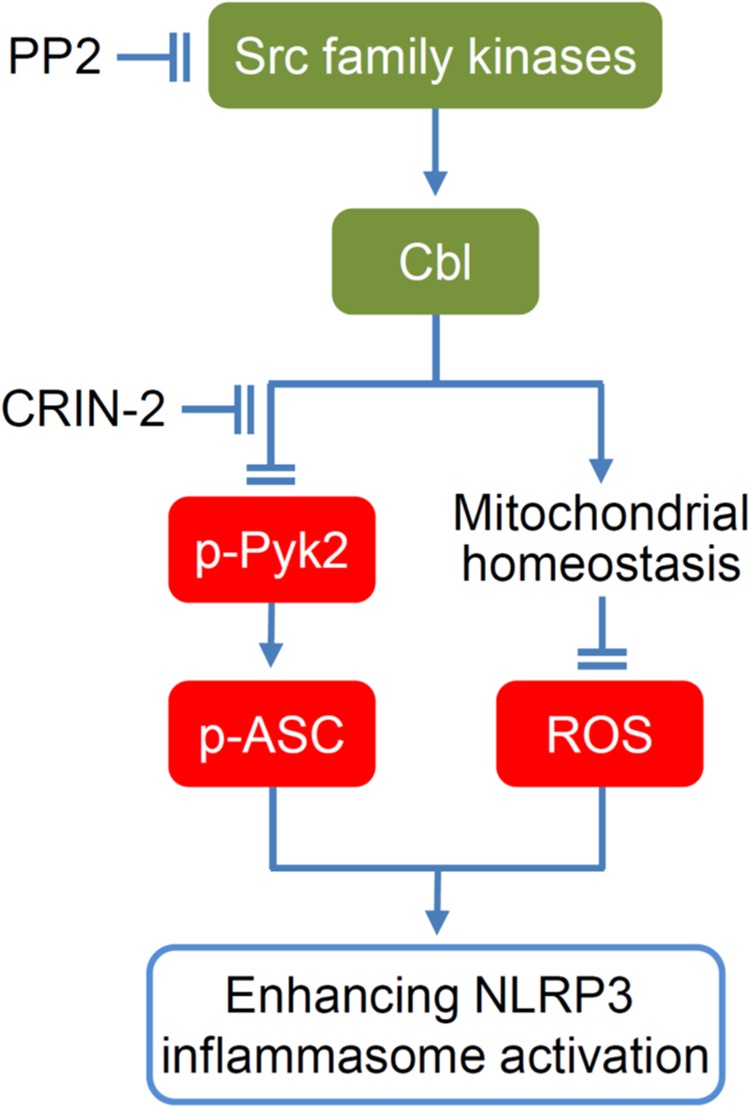
Based on our results, we propose that phosphorylation of Cbl is regulated by Src-family kinases, and that phosphorylation of Cbl at Tyr371 contributes to suppression of the NLRP3 inflammasome. The suppressive function of Cbl is dependent on its ability to downregulate p-Pyk2 via ubiquitination and proteasomal degradation, which reduces the Pyk2-mediated phosphorylation of ASC at Tyr146. Meanwhile, Cbl also reduces mtROS by maintaining homeostasis (appropriate size) of mitochondria. Both Tyr146-phosphorylated ASC and mtROS are negatively regulated by Cbl and are essential for NLRP3 inflammasome activation. Finally, the ability of Cbl to suppress NLRP3 inflammasome activation can be abrogated by the Cbl inhibitor, hydrocotarnine
