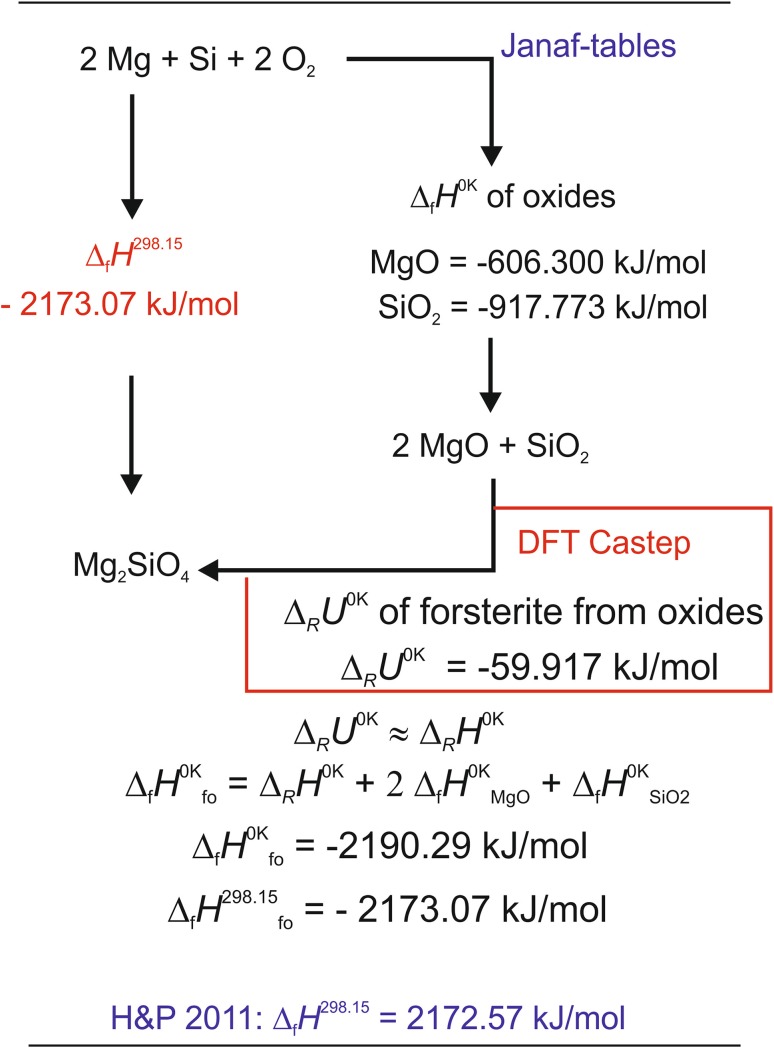
Fig. 1.
Sketch of the calculation procedure for forsterite as an example. The following reaction was investigated: 2MgO + SiO2 = Mg2SiO4. Its reaction energy at 0 K (ΔRU0K) was calculated by the DFT method and it is assumed that the reaction enthalpy at 0 K (ΔRH0K) is identical. Adding the formation enthalpies of the oxides at 0 K (ΔfH0Kox) to this ΔRH0K value results in the formation enthalpy of forsterite from the elements at 0 K (ΔfH0Kfo). The heat content from 0 to 298.15 K (= ∫CPfo dT) is than added to this value yielding finally the standard enthalpy of formation of forsterite from the elements (ΔfH298.15fo)