Abstract
Objectives
Patients with lung cancer (LC) have high rates of psychosocial symptoms and international guidelines recommend regular psychosocial screening during treatment. This study evaluates psychosocial consequences of diagnosis and treatment of LC in a qualitative way and evaluates the need for a LC specific screening instrument.
Methods
Focus group meetings with LC patients were divided by treatment type. Patients discussed psychological and social consequences of diagnosis and treatment. Major themes were identified using content analysis. Themes were re-evaluated in a subsequent focus group, in accordance with the European Organization for Research and Treatment of Cancer (EORTC) guidelines.
Results
Patients reported a range of psychosocial consequences, such as frustration due to physical limitations, fear of recurrence, sadness of leaving behind partner and children, and disappointing social support. Patients treated with palliative intent specifically indicated insecurities about the future. Patients from all treatment modalities indicated a need for family support during treatment. No themes specific to LC arose.
Conclusions
Patients with LC are coping with a range of psychosocial consequences, independent of the type of treatment they receive. Fear of recurrence/metastasis and insecurity about the future were more prominent in patients receiving palliative chemotherapy. Themes were not specific to LC; therefore, a screening instrument specific for the LC population does not seem required. However, the current standard for screening is considered insufficiently sensitive and a stepped screening approach with specific screening tools and a clinical interview is suggested as usual care.
Keywords: Lung cancer, Oncology, Pulmonary, Psychosocial screening, Focus groups, Psychosocial functioning
Background
Lung cancer (LC; non-small-cell lung cancer and small-cell lung cancer combined) is the most common cause of cancer-related deaths worldwide [1]. Global incidence rates are still rising, and in 2020, over 2.2 million new LC patients are expected [2]. In the Netherlands, incidence rates increased with 24.0% between 2000 and 2016 and one in ten newly diagnosed cancer patients in 2016 were diagnosed with LC [3]. This increase is partly due to the aging population. With a 1-year survival rate of 43% and a 3-year survival rate of only 22%, the prognosis of LC is disconcerting.
In the past years, attention for psychological and social consequences of LC has increased and the importance of psychosocial factors in the management of LC has been confirmed [4]. Patients with LC report higher levels of psychosocial problems compared to patients with other types of cancer. Patient-reported depressive feelings varied from 29.5% for gynecological cancer to 43.4% for LC [5], and the incidence of major depression was highest among patients with LC (13.1%) compared to gynecological, breast, colorectal, and genitourinary cancers [6]. LC patients demonstrate higher rates of mixed anxiety/depression symptoms [7]. The prevalence of depression was 43% among 352 patients with small-cell LC and 21% among 366 patients with non-small-cell LC. For anxiety, the prevalence was, respectively, 43 and 25% [8]. Patients with small-cell LC scored significantly higher on both scales. A LC diagnosis predicted clinically relevant scores for symptoms of depression and anxiety 6 months after treatment [9]. These psychological problems seen in patients with LC may result from the poor prognosis and/or demanding treatment trajectories that come with this disease. Cancer-related stigma has also been hypothesized as a possible cause for emotional symptoms in the lung oncologic population [10].
Besides psychological consequences, LC patients are confronted with social consequences of their disease. Compared to patients with other types of cancer (e.g., breast, bowel, prostate, and skin cancers), patients with LC reported more physical and daily living needs (53 vs. 33%). More specifically, they indicated problems with their level of functioning, lack of energy, and pain [11]. Persisting physical symptoms such as fatigue, dyspnea, and pain lead to long-term effects on daily functioning such as ability to work, walking, and self-care [12, 13]. A recent systematic review stated that family-related concerns are common and patients particularly worry about the well-being and coping abilities of their family in relation to caretaking and dealing with their coming death. Moreover, patients exclaimed the importance of social support and reluctance to share their experiences with others because of shame, guilt, and feelings of stigmatization [14].
Based on the established negative psychosocial consequences of cancer, international and national guidelines underline that psychosocial care for patients with cancer is imperative [15, 16]. These guidelines dictate incorporation of psychosocial screening measures in regular care for oncologic patients. Since 2010, these guidelines especially recommend the use of the distress thermometer (DT) together with the problem list (PL) as part of clinical care for cancer patients [16]. The DT-PL is an instrument for routine screening of distress for patients with cancer. Distress is defined as a “multifactorial unpleasant emotional experience of psychological, social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment” [17]. Therefore, the DT-PL evaluates social, psychological, and spiritual/religious aspects of emotional distress [18].
While the DT-PL is an easy to use instrument with good psychometric qualities, the instrument is not cancer-type specific. Consequently, the DT-PL disregards symptoms specific to LC, such as the fear of suffocation or dyspnea. Besides the generic properties of the instrument, the DT-PL only permits dichotomous responses (yes/no), hereby not informing on the extent in which symptoms are present. Finally, the DT-PL lacks a system of referral to compliment the symptom evaluation.
Given the limitations of the current standard for screening in the Netherlands, this study aims to qualitatively examine (disease specific) psychosocial consequences of patients with LC, using focus group methodology. The focus group outcomes will be interpreted in light of the current standard for screening, and suggestions for future screening will be made based on focus group outcomes.
Methods
Procedure
Two focus group sessions were organized. Participants were recruited from March to June 2015 and January to April 2016 from the outpatient clinic of the department of respiratory diseases of the Elisabeth-Tweesteden Hospital Tilburg, the Netherlands.
Session 1: assessment of psychological and social consequences of diagnosis and treatment
The purpose of the first session of focus groups was to explore all psychological and social consequences of diagnosis and treatment of LC. Patients were asked to list all their experiences (physical, environmental, and emotional) after diagnosis and/or treatment. Subsequently, they indicated their personal most important consequence. Thereafter, all input was discussed in the group to identify any other experiences until no new information came up. Finally, patients clustered all input as (1) psychological and (2) social.
Session 2: content analysis and completeness of the issue list
The second session of focus groups aimed to examine the reliability of the themes derived in the first focus group session. In accordance with the guidelines for the development of questionnaires of the European Organization for Research and Treatment of Cancer (EORTC) [19], an issue list was created consisting of the psychological and social themes identified in the first session. An independent LC patient sample evaluated the psychosocial themes derived in the first session and evaluated the completeness of the clusters. Patients were asked to indicate a personal top 10 of the most relevant topics for each cluster (1 = most profound consequence, 10 = least profound consequence). Patients were asked to add any subjective experiences that were missing from the issue list.
Participants
Suitability for focus group participation was estimated by the oncologist (BK), based on clinical outcomes and performance status. The oncologist requested permission for a researcher to contact suitable patients. Hereafter, a researcher screened the medical records of these potential participants. Patients eligible for inclusion were (i) diagnosed with LC and (ii) 18 years or older. Exclusion criteria were (i) inability to attend the focus group meeting, (ii) cognitive problems, (iii) insufficient knowledge of the Dutch language, and (iv) participation in a previous focus group. A researcher contacted eligible patients to explain the purpose of the study, and with permission of the patient, an informative letter and invitation to participate were sent. Participation was voluntary and informed consent was obtained from all individual participants included in the study. Patients did not receive financial compensation, although patients traveling by car received a free parking ticket. All procedures were in accordance with the 1964 Helsinki declaration and its later amendments. Approval from the regional medical ethical committee was obtained (METC/jv/2013.194 protocol no. 1373).
In both focus group sessions, patients were subdivided in focus groups based on the type of treatment they received. This setup allowed for smaller and thus more intimate groups, in which patients had similar treatment conditions. The treatment type was retrieved from the medical records. The first focus group consisted of patients who had completed curative therapy (i.e., surgery or stereotactic radiotherapy). The second focus group consisted of patients with more advanced disease treated with curative intent; these patients had completed chemoradiation therapy. The third focus group consisted of patients receiving palliative chemotherapy during the time of the focus group. The subgroups were invited separately, resulting in three meetings of 90 min in both sessions. A group moderator (MT) guided the meetings in both sessions. In the first session, the moderator was assisted by a research assistant (psychologist in training), and in the second session, a researcher (ML) was present to assist and take notes. Meetings commenced with an explanation of the purpose of the study followed by an introductory round. All patients agreed to the audio recording of the meetings.
Questionnaires
After the focus group meeting, patients completed additional questions concerning sociodemographic information (i.e., age, sex, marital status, educational level).
Data analysis
A grounded theory approach was adopted for analysis of the focus groups (Corbin & Strauss 1990; Glaser & Strauss 1967). Data analysis proceeded stepwise. First, audio recordings were transcribed verbatim. Second, psychological and social consequences were identified as factors and marked by means of open coding. Two authors (ML and MJT) reviewed and coded the transcripts independently to ensure face validity and data saturation. Coding was discussed among these authors. In case of coding disagreement, authors deliberated and selected the most appropriate code. Finally, all the consequences were analyzed using color coding in Microsoft Word and Microsoft Excel. First, the consequences were labeled as psychological or social, and thereafter, similar consequences were clustered into themes. These themes were listed and evaluated in the second focus group session.
Results
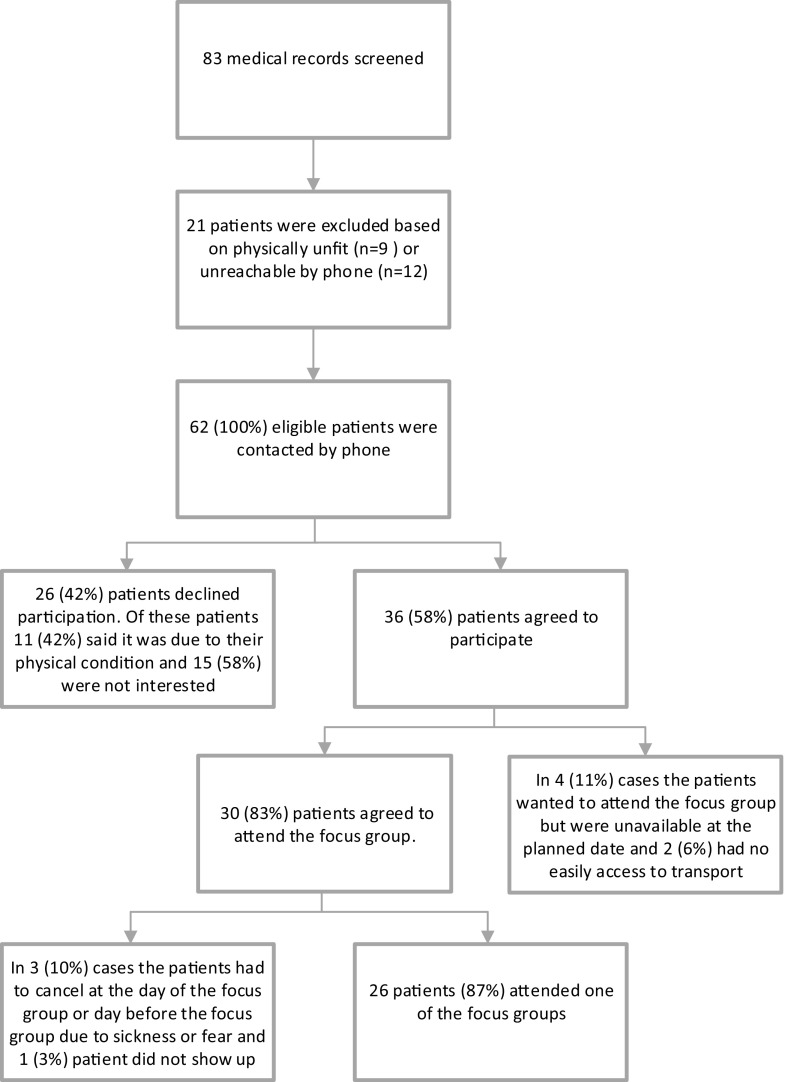
In total, 26 patients participated in this study. Figure 1 displays the study flow. Sociodemographic and clinical characteristics of the sample are presented in Table 1.
Fig. 1.
Study flowchart
Table 1.
Characteristics of the patient sample
| Round 1 (n = 13) | Round 2 (n = 13) | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Age at time of focus group | 63.31 ± 2.68 (49–81) | 66.15 ± 1.96(56–77) |
| Months since diagnosis | 25.23 ± 31.88 (2–124) | 9.85 ± 8.51 (3–36) |
| N (%) | N (%) | |
| Educational levela | ||
| Low | 0 (0%) | 0 (0%) |
| Medium | 8 (61%) | 8 (61%) |
| High | 4 (31%) | 3 (23%) |
| Unknown | 1 (8%) | 2 (16%) |
| Marital status | ||
| Partnered | 12 (92%) | 11 (85%) |
| Widowed/no partner | 1 (8%) | 2 (15%) |
| Diagnosis | ||
| NSCLC | 12 (85%) | 10 (77%) |
| SCLC | 1 (8%) | 3 (23%) |
| Treatment | ||
| Curative therapy (surgery or stereotactic radiotherapy) | 4 (31%) | 4 (31%) |
| Curative intent (chemoradiation therapy) | 3 (23%) | 6 (46%) |
| Palliative chemotherapy | 6 (46%) | 3 (23%) |
aLow = < 10 years of education, medium = 10–14 years of education, high = > 14 years of education
NSLC non-small-cell lung cancer, SCLC small-cell lung cancer
Session 1: assessment of psychological and social consequences of diagnosis and treatment
An excerpt of the patient-reported psychological and social consequences is presented in Table 2. There were small differences between the subgroups, and results specific to a treatment group are explicitly reported.
Table 2.
Excerpt of patient-reported psychological and social experiences of diagnosis and treatment
| Consequences | Themes | Specification | Quotes |
|---|---|---|---|
| Psychological | Acceptation of physical limitations | Acceptation over time Ongoing frustrations/non-adaptation |
“Well, there are things you cannot do anymore, but that also has psychological consequences, like you say damn it, I cannot do things anymore.” “Getting a little angry that it’s eh, actually not possible anymore” |
| General anxiety | Fear of pain caused by treatment Fear of living with limitations Fear of cancer |
“For when they hurt me, and I am just very scared. When I walk into this hospital, I think it is frightful” “Yes, that is just my fear. I will not return to my old self” “Yes, I worried a lot during that time, I lay awake from is, I hardly slept at all. I got up and it felt just like I had worked all day, to say it like that. It was not pleasant” |
|
| Fear of recurrence/deterioration | Fear of medical examinations Fear caused by physical sensations |
“Yes, anxious. When you have that picture taken and you have to wait for the results... That will obviously stay with me” “… the first moment you feel something again, the first thing that pops up in your mind is: shit.” |
|
| Increased emotionality | Overall more emotional Anger Worrying Sadness and feeling down |
“I am just a lot more emotional, when I see something on TV I can cry about it. And also when I talk about it.” “Yes, certainly in the beginning. There was little to be said, but then BAM” (agitation) “With cancer it comes with so many thoughts, that you keep thinking; with what could that have to do?” “Not every 30 min, there are days that absolutely nothing happens, and every now and then I feel down” |
|
| Guilt about changed family roles | Not being able to work | “Because I cannot do what I could do anymore, to say it in a popular way… I was breadwinner, and now I am the boarder, you see?” | |
| Insecurity about the future | Not knowing how long to live | “…you can better live with knowing I have 4 months left, in that case you know you have a 100% energy, you can work through your bucket list and it is done. But now it is like, will I hang the Christmas balls in the tree? Will I see the little ducks swim in the water next year?” | |
| Shock of diagnosis | Unexpectedness of diagnosis resulted in fear and irritations Acceptance |
“Yes, I had not been sick before, that was the strange part about it... and all of a sudden bam...” “To be honest, that struck me hard, I felt the world fall from under my feet. But that was only for a short period of time, although for me it was a short time. It just is what it is, I know what I have and I know that nothing can be done except one chemotherapy, and eh than there is one option and that is to stay positive in life and keep working, that I still do.” |
|
| Social | Influence on family | Fear Shock Insecurity Bringing family together |
“I can do less and that also has consequences for my family and I think that’s highly bothersome to say it like that, I feel really lousy about it.” |
| Response from social environment | Being treated as a patient Fear and uneasiness Mixed response and finding out who your real friends are Positive responses and support Feeling judged for smoking |
“There are a few people who are extra caring, but there are also some who leave you” “Do you not have people in your surroundings that still think: Will they be contagious? We’d rather not go there...” “There are people, from whom I know that they know, who do not dare to ask anything. Who just give a wide berth walk.” “There are some people they accept it like me, like yes, I am happy that you are still here and we have to keep on going, other people immediately think you are an idiot” “Especially by non-smokers, they easily judge you, like I do not see how you still dare to smoke. Yes...” |
|
| Attention to family system | Insecurity Anger Adaptation of family system Coping of children Shock for partners |
“But I think that is also the most difficult for the family. Indeed attention of friends and acquaintances but also in the hospital, all attention goes to you (the patient). While they actually sit around a little bit and for them it is actually just as bad.” “Well, actually, when you get something like this. It is worse for the children and wife I think, than it was for myself.” |
|
| Financial consequences | Burden of decreased income Cost of physiotherapy |
“…that will be a burden because I am going low in income or at least incapacitated when I am home for more than half a year. A lot of travel costs with 33 times radiation; you have to go to Tilburg 3 times. It’s only a few minutes but you just do have to go to Tilburg and back.” | |
| Work consequences | Effect of LC on work relations | “During conversations with the psychologist I came to the conclusion that, in my case, it is more in the work environment, than in the consequences of my disease. That had a big impact on me, I honestly have to say.” |
Psychological themes
Addressing the time of diagnosis, patients identified “shock of diagnosis” as a theme. Two patients reported that their cancer had been discovered during an examination for a comorbid condition. To them, the diagnosis came as a great shock because they did not feel sick or present limiting physical symptoms at that time. One patient emphasized his anger in reaction to the diagnosis because he had never smoked. Positive adaptation after diagnosis was specified within this theme by five patients in the palliative treatment, as they described acceptation of the diagnosis and a changed perspective on daily life.
“Increased emotionality” was a common theme among groups. Patients reported crying more often and feeling sad when exposed to sadness. This emotionality also occurred in reaction to non-cancer-related triggers, for example, when confronted with sadness on television or in their surroundings.
Acceptation of physical limitations’ was identified as a theme. Patients either reported ongoing frustrations such as fear, anger, and sadness caused by physical limitations, or acceptation of the physical state over time. Two patients expressed feelings of guilt towards their family because they could not participate and contribute to the household as they used to. “Insecurities about the future” was identified as a theme, especially by patients receiving palliative treatment. One patient thought that it would be easier to know that he had only several months left than having to live with the insecurity of not knowing how much time he had. Another patient expressed the opposite opinion and did not want his doctor to share information about the end of life with him.
“Fear of recurrence and/or metastasis” of the cancer was discussed in all groups, although this fear was most explicitly present in groups treated with chemoradiation and palliative chemotherapy. One patient in the palliative treatment group mentioned anxiety for diagnostic procedures and the pain they might cause. Patients treated with curative intent reported heightened awareness of physical symptoms. A cold, pain, or vague physical discomfort could trigger fear of recurrence/metastasis in this group. Finally, “general anxiety” was identified as a theme, as patients in all groups reported increased anxiety and nervous sensations in the days prior to medical checks. This fear was attributed to pain and the possibility of bad results. Patients described that fear of recurrence/metastasis faded somewhat in time because of positive medical checks.
Social themes
Patients identified “influence of LC on the family” as a social theme. In every group, patients declared that their partners and children had a hard time dealing with the diagnosis and the disease, causing feelings of sadness, anger, or frustration in partners and patients. Patients mentioned changed dynamics in the family caused by LC, e.g., inability to work, time spent with the family, or inability to perform household tasks. These changes sometimes caused irritations in both patients and partners. One patient felt that he interfered with the daily routine, causing difficulties and irritations with his partner. Patients indicated that one of the hardest things was to see the sadness that the cancer induced in their children. Patients agreed with each other that cancer is not an individual disease, but that it greatly affects the family. Two patients remarked that health care professionals and the support system tended to focus on the patient, rather than the well-being of other members of the family. All groups emphasized the importance of attention and support for other family members, resulting in the theme “attention to the family system.”
The theme “response from social environment” was identified as patients from all groups noticed changes in relationships due to their cancer. Two patients said they had discovered the value of friendships and felt that they grew closer to people close to them. Six patients had negative experiences, in which friends and acquaintances avoided conversations about their LC or reduced contact altogether. Other negative social consequences were being treated as a weak/sick person, and decreased support with the passing of time (e.g., people moving on with their own lives after the initial shock of diagnosis had passed). Patients who continued smoking after diagnosis mentioned feeling judged by non-smokers. Two of four patients who were still working experienced negative “consequences in the work environment,” as they felt less involved. Two patients described “financial consequences” of LC, resulting from decreased income or high costs for physiotherapy.
Session 2: content analysis and completeness of the issue list
Patients recognized all the consequences identified in session 1 and no additional consequences came up in the second session. Fear of recurrence was ranked among the three most important psychological consequences in seven out of ten patients treated with curative intent. All patients receiving palliative treatment indicated sadness about leaving behind partner/children as one of the most important psychological consequences of disease.
Conclusion
This study is among the first to qualitatively examine psychosocial experiences of patients with LC during/after diagnosis and treatment. “Increased emotionality,” “acceptation of physical limitations,” “general anxiety,” and “fear of recurrence” were considered the most important psychological consequences. Patients receiving palliative chemotherapy specifically mentioned “insecurity about the future” as an important psychological theme of their cancer. With regard to the social consequences, most patients reported the major influence of the disease on their partner and/or children and the changed family dynamics. Consequently, patients emphasized a need for family support from both health care professionals and the social environment. This was reflected by the themes “influence on family” and “attention to family system.” It was evident that patients are coping with a range of “response from social environment,” as this was identified as the third major social theme.
There were some differences between patients from different treatment modalities, especially the theme “insecurity about the future” that was characteristic for the palliative treatment group. When comparing these outcomes of patients with LC to reported consequences of patients with other types of cancer, the psychosocial themes identified in the focus groups are similar to experiences of patients with other types of cancer [20]. This suggests that a LC specific screening instrument is not required and screening instruments for cancer patients in general can adequately estimate psychosocial symptoms of patients with LC. In the Netherlands, the DT-PL is regarded the golden standard for distress screening for patients with cancer. For general complaints, the DT-PL and its dichotomous character may be sufficiently sensitive. There has been some criticism on the DT-PL, as the DT-PL does not indicate the gravity of symptoms and overlooks detailed psychosocial experiences such as those identified as most relevant in this study. Different types of fears/worries were specified among the most important psychological themes in this study. Patients with other cancers have also described fear of pain caused by treatment, fear of recurrence/metastasis, and fear of not achieving physical recovery [21, 22]. The DT-PL addresses these experiences with a single item (“anxiety”). A screening procedure based solely on the DT-PL therefore disregards the most prominent themes in our sample.
To facilitate a more extensive exploration of psychological symptoms that are only addressed in the DT-PL with a single item (e.g., sadness or anxiety), a second-stage screening method containing multiple, complementary generic instruments and a clinical interview is suggested [23, 24]. The Patient Health Questionnaire Nine-Symptom Depression Scale (PHQ-9) [25] and the Generalized Anxiety Depression Scale (GAD-7) [26] have been recommended as second-stage screening instruments, supplemented by a clinical interview by a licensed mental health professional [23, 24]. The interview allows for personalized, adaptive screening (e.g., to fear for treatment/recurrence/metastasis or the nature of relational problems). Conjointly, these elements form a semi-structured psychosocial screening method in which the DT-PL functions as a conversation starter, a first step to comprehending psychosocial experiences [27], and any psychological symptoms of anxiety or depression are screened more comprehensively.
There is evidence that this type of stepped health care can function as a source of support for patients with a lack of social support, thereby promoting quality of life [28]. Although the screening procedure itself can be therapeutic, some patients require complementary psychological or supportive care. Since referral to psychosocial health services was the best predictor of decreased anxiety [29], the lack of a referral system is considered another shortcoming of the DT-PL. In the stepped screening approach, the clinical cutoff scores employed in the second step screening instruments and the clinical interview aid subsequent referral. In absence of an evidence-based system of referral, training staff to use and interpret a psychosocial screening instrument will aid the referral process [30].
In addition to their own experiences, patients in all treatment groups stressed the importance of support for family members during the trajectory. The interrelatedness of the well-being of oncologic patients and their partners underlines the importance of family support [31]. Family therapy and problem-solving techniques are known to positively affect the well-being of caregivers of patients with cancer [32]. Individual treatment could help partners to disclose insecurities and increase positive appraisal [33]. Further research could evaluate the possibilities for family support during treatment for LC. The financial structure of health care constitutes a practical barrier for the implementation of family support programs/therapies in Dutch hospital care. Therefore, health care professionals should raise awareness for the possibilities for family support provided outside the hospital, for instance, in the clinical interview as part of the stepped screening procedure.
Study limitations
A strength of this study is the semi-structured qualitative study design, facilitating in-depth exploration of psychosocial consequences of diagnosis and treatment of LC. Patients seemed to feel free to share their experiences, resulting in in-depth conversations. Interviews were conducted in a standardized manner, resulting in clustered information and a consistent style of questioning. There are some points of improvement. As patients were selected by their oncologist, the selection procedure allowed for selection bias. Patients with poor physical condition did not participate in the focus groups, possibly resulting in an underrepresentation of psychosocial consequences specific to LC. For example, dyspnea is highly prevalent among patients with poor clinical performance and often causes anxiety and fear of suffocation [34]. This topic should be incorporated in the screening procedure, for instance, in the clinical interview. With a response rate of 58%, there is a possibility of response bias. Patients with an avoidance coping strategy are more likely to refuse participation, and the relevance of psychosocial complaints might decrease over time. Participants were diagnosed on average 17.5 months before the interview, resulting in a possible underrepresentation of LC specific consequences in our sample. The characteristics of the patients in both focus group rounds were not exactly the same; i.e., groups differed with regard to time since diagnosis. In general, this is undesirable because a good representation of patients is wanted in all groups. Fortunately, in the current study, patients in the second round, who had a shorter time since diagnosis, did not suggest any new themes. Of the patients who declined participation, 58% indicated that they were not interested or felt uncomfortable talking to strangers about health or psychosocial problems. To conclude, the study sample consisted of Caucasian participants exclusively, and studies are required to examine psychosocial consequences of LC in patients with other ethnic backgrounds.
Clinical implications
Patients with LC face many different psychosocial challenges, which are insufficiently detected by the current screening procedures. Early detection of psychosocial complaints is imperative in order to provide adequate psychosocial care or facilitate referral to other care professionals (e.g., medical psychologist). Although this study did not indicate a need for a LC specific screening instrument, the current standard for screening still leaves room for improvement as common reported problems are regularly overlooked. Consequently, a stepped care screening method is warranted. A general screening instrument, such as the DT-PL, complemented by specific second-stage screening instruments and a personalized clinical interview (where one can also address issues such as fear of suffocation if needed) will aid health care professionals by providing an overview of the symptoms and their gravity and substantiate adequate referral. A stepped care screening method contributes to comprehensive and personalized psychosocial support for patients with LC.
Acknowledgements
We would like to thank patients of the pulmonary ward of the Elisabeth-TweeSteden Hospital for sharing their experiences. This project received partial funding of the Shared Decision Making research initiative of CZ health insurance company in the Netherlands. The funder was not involved in the execution of this study and did not influence scientific integrity of anyone involved.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al.(2013) GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase no. 11 [internet]. Lyon, France: International Agency for Research on Cancer; . Available from: http://globocan.iarc.fr, accessed June 2017
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray, F. (2013). GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; Available from: http://globocan.iarc.fr, accessed on 25/08/2017
- 3.Integraal Kankercentrum Nederland. Dutch Cancer Figures http://www.cijfersoverkanker.nl/ [cited; 2016 02]
- 4.Leduc C, Antoni D, Charloux A, Falcoz P, Quoix E. Comorbidities in the management of patients with lung cancer. Eur Respir J. 2017;49:1601721. doi: 10.1183/13993003.01721-2016. [DOI] [PubMed] [Google Scholar]
- 5.Zabora J, Brintzenhofe-Szoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psycho-Oncology. 2001;10(1):19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::AID-PON501>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Walker J, Hansen C, Martin P, Symeonides S, Ramessur R, Murray G, Sharpe M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry. 2014;1(5):343–350. doi: 10.1016/S2215-0366(14)70313-X. [DOI] [PubMed] [Google Scholar]
- 7.Brintzenhofe-Szoc K, Levin T, Li Y, Kissane D, Zabora J. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics. 2009;50(4):383–391. doi: 10.1176/appi.psy.50.4.383. [DOI] [PubMed] [Google Scholar]
- 8.Hopwood P, Stephens R. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18(4):893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 9.Boyes A, Girgis A, D’Este C, Zucca A, Lecathelinais C. Prevalence and predictors of the short-term trajectory of anxiety and depression in the first year after a cancer diagnosis: a population-based longitudinal study. J Clin Oncol. 2013;31:2724–2729. doi: 10.1200/JCO.2012.44.7540. [DOI] [PubMed] [Google Scholar]
- 10.Chambers S, Baade P, Youl P, Aitken J, Occhipinti S, Vinod S, Valery P, Garvey G, Fong K, Ball D, Zorbas DJ, O’Connel D. Psychological distress and quality of life in lung cancer: the role of health-related stigma, illness appraisals and social constraints. Psycho-Oncology. 2015;24(11):1569–1577. doi: 10.1002/pon.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Girgis A. Supportive care needs: are patients with long cancer a neglectved population? Psycho-Oncology. 2006;15:509–516. doi: 10.1002/pon.983. [DOI] [PubMed] [Google Scholar]
- 12.Hung R, Krebs P, Coups E, Feinstein M, Park B, Burkhalter J, Ostroff J. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. J Pain Symptom Manag. 2011;41(2):426–435. doi: 10.1016/j.jpainsymman.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanake K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advances lung cancer. J Pain Symptom Manag. 2002;23(5):417–423. doi: 10.1016/S0885-3924(02)00376-7. [DOI] [PubMed] [Google Scholar]
- 14.Maguire R, Papadopoulou C, Kotronoulas F, Simpson M, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17(4):449–464. doi: 10.1016/j.ejon.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen P, Wagner L. A new quality standard: the integration of psychosocial care into routine cancer care. J Clin Oncol. 2012;30(11):1154–1159. doi: 10.1200/JCO.2011.39.5046. [DOI] [PubMed] [Google Scholar]
- 16.Oncoline. Screening for psychological distress. Retrieved from: http://www.oncoline.nl/screening-for-psychological-distress [cited; 2016 02]
- 17.NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Distress Management version 3.2012.2012. Available from: http://www.nccn.org/professionals/physican_gls/f_guidelines.asp
- 18.Tuinman M, Gazendam-Donofrio S, Hoekstra-Weebers J. Screening and referral for psychosocial distress in oncologic practice: use of the distress thermometer. Cancer. 2008;113(4):870–878. doi: 10.1002/cncr.23622. [DOI] [PubMed] [Google Scholar]
- 19.Group EQoL. Guidelines for Developing Questionnaire Modules 2011 [05–10-2016]. Available from: http://groups.eortc.be/qol/sites/default/files/archives/guidelines_for_developing_questionnaire-_final.pdf
- 20.Stanton A. Psychosocial concerns and interventions for cancer survivors. J Clin Oncol. 2006;24(32):5132–5137. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 21.Curran L, Sharpe L, Butow P. Anxiety in the context of cancer: a systematic review and development of an integrated model. Clin Psychol Rev. 2017;56:40–54. doi: 10.1016/j.cpr.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Herschbach P, Keller M, Knight L, Brandl T, Huber B, Henrich G, Marten-Mittag B. Psychological problems of cancer patients: a cancer distress screening with a cancer-specific questionnaire. Br J Cancer. 2004;93(3):504–511. doi: 10.1038/sj.bjc.6601986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen B, DeRubeis R, Berman B, Gruman J, Champion V, Massie M, Holland J, Partridge A, Bak K, Somerfield M, Rowland J. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605–1619. doi: 10.1200/JCO.2013.52.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen B, Rowland J, Somerfield M. Screening, assessment, and Care of Anxiety and Depressive Symptoms in adults with Cancer: an American Society of Clinical Oncology guideline adaptation. J Oncol Pract. 2015;11(2):133–134. doi: 10.1200/JOP.2014.002311. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer R, Kroenke K, Williams J, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 27.Lynch J, Goodhart F, Saunders Y, O’Connor S. Screening for psychological distress in patients with lung cancer: results of a clinical auit evaluating the use of the patient distress thermometer. Support Care Cancer. 2011;19(2):193–202. doi: 10.1007/s00520-009-0799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luszczynska A, Pawlowska I, Cieslak R, Knoll N, Scholz U. Social support and quality of life among lung cancer patients: a systematic review. Psycho-Oncology. 2013;22(10):2160–2168. doi: 10.1002/pon.3218. [DOI] [PubMed] [Google Scholar]
- 29.Carlson L, Groff S, Maciejewski O, Bultz B. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. J Clin Oncol. 2010;28(33):4884–4891. doi: 10.1200/JCO.2009.27.3698. [DOI] [PubMed] [Google Scholar]
- 30.Carlson L, Waller A, Mitchell A. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160–1177. doi: 10.1200/JCO.2011.39.5509. [DOI] [PubMed] [Google Scholar]
- 31.Edwards B, Clarke V. The psychological impact of a lung cancer diagnosis on families: the influence of family functioning and patients'illness characteristics on depression and anxiety. Psycho-Oncology. 2004;13:562–576. doi: 10.1002/pon.773. [DOI] [PubMed] [Google Scholar]
- 32.Applebaum A, Breitbart W. Care for he cancer caregiver: a systematic review. Palliat Support Care. 2013;11(3):231–252. doi: 10.1017/S1478951512000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Northouse L, Katapodi M, Song L, Zhang L, Mood D. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin. 2010;60(5):317–339. doi: 10.3322/caac.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Driscoll M, Corner J, Bailey C. The experience of breathlessness in lung cancer. Eur J Cancer Care. 1999;8(1):37–43. doi: 10.1046/j.1365-2354.1999.00129.x. [DOI] [PubMed] [Google Scholar]