Abstract
Purpose
Riluzole – a major therapeutic medicine for patients with amyotrophic lateral sclerosis – reportedly has anti-nociceptive and anti-allodynic efficacies in neuropathic pain models. However, little is known about its effect on neurotransmission in the spinal superficial dorsal horn (SDH). The present study aims to investigate the effects of riluzole on the synaptic transmission of SDH nociceptive pathways in both physiological and pathological conditions.
Materials and methods
Spinal nerve ligation was used to produce a neuropathic pain model. Mechanical allodynia behavior was assessed with Von Frey filaments. Riluzole’s effects on nociceptive synaptic transmission under both physiological and pathological conditions were examined by patch-clamp recordings in rat SDH neurons.
Results
The principal findings of the present study are three-fold. First, we affirm that riluzole has a remarkable long-lasting analgesic effect on both in vitro and in vivo pathological pain models. Second, the prolonged inhibitory effects of riluzole on spinal nociceptive signaling are mediated by both presynaptic and postsynaptic mechanisms. Finally, endocytosis of post-synaptic GluR2 contributes to the riluzole-induced long-term depression (LTD) of the spinal nociceptive pathway.
Conclusion
The present study finds that riluzole induces LTD of nociceptive signaling in the SDH and produces long-lasting anti-allodynia effects in nerve injury-induced neuropathic pain conditions via postsynaptic AMPA receptors associated with the endocytosis of GluR2.
Keywords: riluzole, neuropathic pain, superficial dorsal horn, SSDH, long-term depression, LTD, AMPA receptor endocytosis
Introduction
The spinal superficial dorsal horn (SDH) is involved in the transmission and modulation of primary nociceptive inputs.1–3 It is well-known that peripheral nerve injury impairs the balance between inhibitory and excitatory synaptic functions in the SDH, leading to the induction and maintenance of neuropathic pain.4 Unfortunately, effective treatment options for patients with neuropathic pain are few.
Riluzole is a unique neuroprotective drug for treating amyotrophic lateral sclerosis (ALS).5,6 In addition, the neuroprotective effects of riluzole have been reported in spinal cord injury7,8 and neurodegenerative disorders.9,10 Riluzole has demonstrated remarkable antinociceptive effects in neuropathic pain animal models.6,11–13 In a spinal cord injury animal study, riluzole that was injected into the locus coeruleus increased the thermal paw withdrawal latency and the paw mechanical withdrawal threshold.14
Riluzole reportedly has an analgesic effect on neuropathic pain, but little is known about its effect on synaptic transmission in the spinal SDH. The current experiments aimed to explore the actions of riluzole on synaptic transmission in the SDH. Our findings indicated that riluzole inhibited excitatory synaptic transmission in the SDH via both presynaptic and postsynaptic mechanisms. We further found that riluzole induced long-term depression (LTD) of synaptic transmission from primary Aδ and C fibers to SDH neurons via altered efficiency of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.
Materials and methods
Animals
Male Sprague Dawley rats (21–35 days old) used in the experiments were obtained from the Animal Center of the Fourth Military Medical University. All animal experiments were conducted according to the recommendations of IASP and the NIH Guide for the Care and Use of Laboratory Animals. All animal procedures were approved by the Ethics Committee for Animal Experimentation of the Fourth Military Medical University (Xi’an, China). The animals were raised in a temperature-controlled room with controlled lighting (06:00–18:00) and supplied with laboratory chow and water.
Preparation of spinal cord slices
Rats were anesthetized by intraperitoneal (i.p.) injection of pentobarbital (40–60 mg/kg) and perfused with sucrose-substituted artificial cerebral spinal fluid (sucrose ACSF; 75.00 mM sucrose; 80.0 mM NaCl; 2.50 mM KCl; 2.50 mM CaCl2; 1.20 mM MgCl2; 1.25 mM NaH2PO4; 25.00 mM NaHCO3; 1.30 mM ascorbate; and 3.00 mM pyruvate, aerated with 95% O2 and 5% CO2 for 30 minutes and stored at 4°C) for 2 minutes through the aorta after exposing the apex cordis. The lumbar segment was dissected and fixed in sucrose ACSF quickly. The parasagittal spinal slice (450–500 µm thick) with an attached dorsal root (0.8–1.0 cm long) was cut with a vibrating microtome and was incubated with Glucose ACSF (125.00 mM NaCl; 2.50 mM KCl; 2.00 mM CaCl2; 1.00 mM MgCl2; 1.25 mM NaH2PO4; 26.00 mM NaHCO3; 25.00 mM d-glucose; 1.30 mM ascorbate; and 3.00 mM pyruvate, aerated with 95% O2 and 5% CO2) at room temperature (22°C–25°C, 35 mL/min).15
Electrophysiological recordings
The procedures for patch-clamp recordings have been previously documented.15 Current- or voltage-clamp recordings were made from neurons in lamina II of the SDH with patch-pipette electrodes (5–10 MΩ), using an Axon 200B amplifier at room temperature (25°C). The composition of the pipette solution was described in a previously published article.15 The Digidata 1,200 interface and pCLAMP 10 software (Axon Instruments) were used to acquire and analyze the data. The holding potentials (HPs) were set at −70 mV in voltage clamp mode to preserve pure excitatory postsynaptic currents or clamped at −50 mV in current clamp mode to reveal mixed excitatory and inhibitory synaptic events.
A suction electrode was utilized to irritate the dorsal root (DR, 6–10 mm long) to evoke excitatory postsynaptic currents (eEPSCs) or potentials (eEPSPs) in lamina II neurons. Repetitive DR stimulation (20 Hz for A fiber, 1 Hz for C fiber) was used to distinguish monosynaptic or polysynaptic eEPSC(P)s. Monosynaptic eEPSC(P)s typically had constant latencies without synaptic failure.16,17 A combination of response threshold and conduction velocity was used to determine the classification of Aδ and C fibers mediating the synaptic responses.15 The average amplitude of 20 eEPSC(P)s acquired before, during, and after drug application was compared. Miniature EPSCs (mEPSCs) were analyzed with Mini-Analysis 5.0 to compare the cumulative probability of mEPSC amplitude and inter-event intervals.
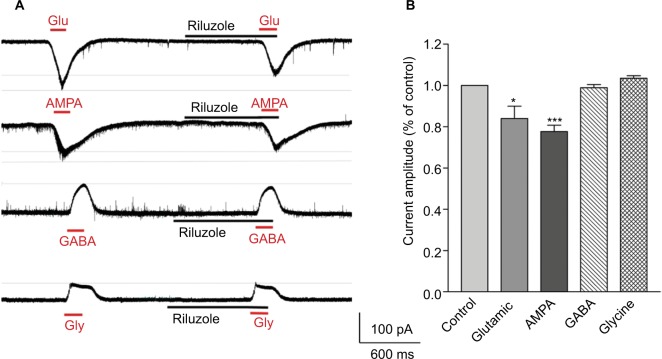
Drug-evoked membrane currents were recorded in the gap-free mode. The slices were perfused with glutamate (Glu; 100 µM), AMPA (100 µM), gamma-Aminobutyric acid (GABA; 3 µM), and glycine (Gly; 30 µM), respectively, to induce the membrane current, and were then washed with ACSF. The membrane currents evoked by Glu, AMPA, GABA, and Gly were recorded again during the perfusion with riluzole (50 µM, 2 minutes).
We used an in vitro hyperexcitability model in which a mixture of Gly receptor antagonist strychnine (2 µM) and GABAA receptor antagonist bicuculline (20 µM) was perfused onto the slices to assess the effect of riluzole under the experimental pathological conditions.
Western blotting
Adult male Sprague Dawley rats (180–220 g) were deeply narcotized by an i.p. injection of pentobarbital (40–60 mg/kg), and the chest was exposed to intubate the left ventricle to the aorta. The right atrium was cut and opened. Rapid perfusion with 100 mL 4°C sucrose ACSF rinsed the blood through the aorta. Fresh tissue samples from the L5 spinal cord dorsal horns were removed as quickly as possible at low temperature. We extracted spinal cord complete protein according to the steps defined in the Millipore membrane protein extraction kit manual by using a low-temperature homogenizer and boiled the sample for 10 minutes. A bicinchoninic acid protein quantification kit was used to determine the protein concentration. Protein samples (15 µg) were separated on an SDS–PAGE gel (12.5%) and transferred to polyvinylidene difluoride filters. Then they were blocked with 5% tris-buffered saline with tween (TBST) skim milk for 1 hour and incubated overnight at 4°C with a rabbit anti-GluR2 primary antibody (1:1,000; Millipore, Billerica, MA, USA) and a mouse anti-N-cadherin TBST antibody (1:4,000; Millipore), and then rinsed three times (5 minutes each time) with TBST. The blots were incubated for 1 hour at room temperature with the pertinent secondary antibody horseradish peroxidase-conjugated anti-rabbit IgG (1:20,000; Zhongshan Jinqiao Biotechnology Co, Beijing, China), and photographed with a gel imaging analysis system (Alpha Innotech, FluorChem, FC2); thereafter, the protein bands were analyzed.
Spinal nerve ligation model
Rats were anesthetized with pentobarbital. The lumbar 5 (L5) spinal nerve ligation (SNL) was conducted as previously described.15,18,19 In brief, the L5 nerve was isolated after removal of the spinal transverse process. Subsequently, the L5 nerve was tightly ligated with 5–0 silk thread twice at 0.3-cm intervals. Then, the incision was sutured layer by layer. After the surgery, all animals were transmitted to a feeding room, where they were closely monitored to recover from the operation. Control surgery followed the same procedure except for the ligation of the L5 spinal nerve.
Assessment of mechanical allodynia behavior
Following the double-blind principle, behavioral tests were conducted by a blinded observer. Rats were adapted to the testing environment for three successive days before the surgery. The behavior tests were conducted in a quiet room (23±1)°C from 9:00 am to 12:00 am.
The incidence of foot withdrawal evoked by mechanical stimulation to the hind-foot plantar surface by Von Frey filaments (2–17.0 g; North Coast Medical, Inc.) was recorded to determine the paw withdrawal mechanical threshold (PWMT).20 Briefly, animals were housed in a diaphanous plastic box (30×30×30 cm) with a metal mesh bottom. After 15 minutes of acclimatization, a filament was applied through the mesh to the hind-foot plantar surface. The stimulation lasted for 2–3 seconds, repeated at 15-second intervals. The percentage of foot withdrawal in ten stimulations was recorded.
Pharmacological administration
The drugs applied in this experiment were riluzole, bicuculline, strychnine, Glu, AMPA, GABA, Gly, and Tat-GluR2. All compounds were purchased from Sigma, except GABA which was procured from Tocris Cookson. Riluzole was dissolved in dimethyl sulfoxide (DMSO) for preservation. SNL rats were given i.p. injection of riluzole (12 mg/kg) or vehicle (DMSO in 0.9% saline 2 mL) at 5 days post SNL surgery.
Statistical analysis
The data obtained were recorded as mean ± SEM. Paired t-tests, non-paired t-tests, K–S tests, or one-way AVOVA were used to test the changes of values for the verification of significance. Unpaired Student’s t-test or repeated-measures ANOVA followed by post hoc Bonferroni tests were used to analyze the behavioral data. P-values <0.05 were considered statistically significant.
Results
Riluzole inhibits excitatory synaptic activities in SDH by both presynaptic and postsynaptic mechanisms
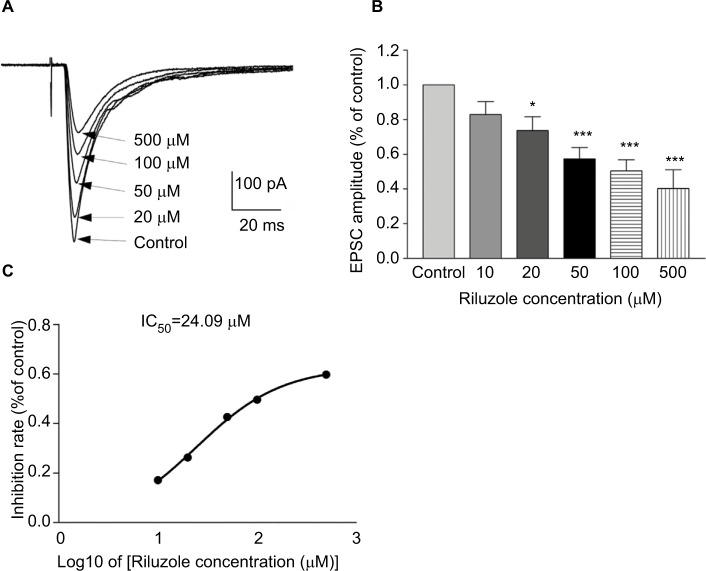
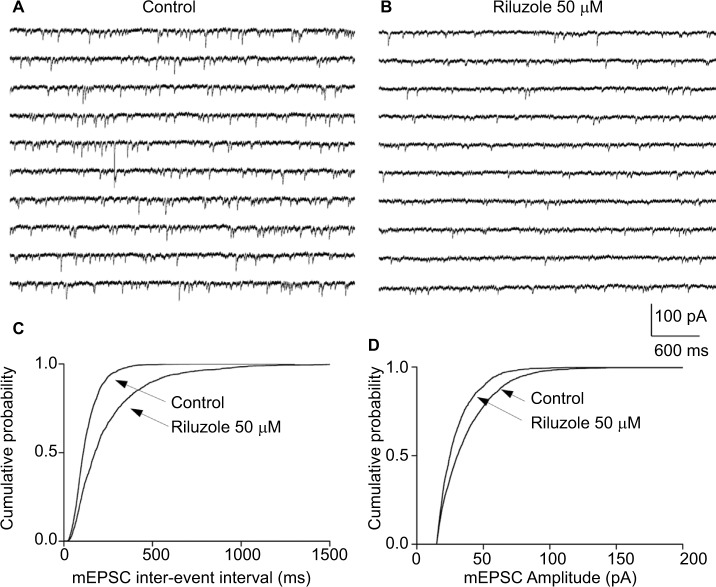
The spinal SDH plays an important role in pain modulation, in which primary sensory inputs are received, integrated, and ascended to higher brain regions.1–3 We first tested the actions of riluzole on excitatory synaptic inputs to neurons in lamina II from C and Aδ fibers. As shown in Figure 1A, DR stimulation evoked a reproducible monosynaptic eEPSC in the lamina II neurons recorded (n=89). The conduction velocity (CV) of the primary input fibers responsible for the monosynaptic responses varied from 0.3 to 2.2 m/s – within the limits of CV of Aδ (1.5–2.2 m/s) and C (0.3–0.5 m/s) fibers.21 Riluzole was bath-applied by superfusion for 30 seconds at different concentrations (10–500 µM). The amplitude of both C and Aδ fiber-evoked monosynaptic eEPSCs were significantly decreased by riluzole. Compared with the control, superfusion of 10, 20, 50, 100, and 500 µM riluzole decreased the amplitudes to 82.85%±7.53% (P<0.05, n=6), 73.6%±7.95% (P<0.05, n=6), 57.36%±6.61% (P<0.001, n=6), 50.35%±6.53% (P<0.001, n=6), and 40.23%±10.78% (P<0.001, n=4), respectively (Figure 1B). The IC50 was calculated as 24.09 µM (Figure 1C). Notably, the inhibitory effect of riluzole irreversibly lasted for at least 60 minutes and throughout the entire observation period (Figure 2). Furthermore, the effect of riluzole on the miniature EPSCs (mEPSCs) was tested in lamina II neurons. As seen in Figure 3, both the amplitude and frequency of mEPSCs were reduced by 50 µM riluzole (P<0.05, n=8). The above experiments suggest that riluzole effectively inhibited excitatory synaptic transmission from primary C and Aδ afferent fibers to certain spinal lamina II neurons via both presynaptic and postsynaptic mechanisms.
Figure 1.
Riluzole inhibits excitatory synaptic transmission in a concentration-dependent manner.
Notes: (A) Traces of eEPSCs in SDH neurons in the presence of different concentrations of riluzole (20, 50, 100, and 500 µM). (B) The histogram indicates the monosynaptic eEPSC peak amplitude in different concentrations of riluzole compared with control (mean ± SEM *P<0.05, ***P<0.001, paired t-test). HPs were clamped at −70 mV. (C) Dose–response curves for IC50 of riluzole.
Abbreviations: eEPSC, evoked excitatory postsynaptic currents; IC50, half maximal inhibitory concentration; SDH, superficial dorsal horn.
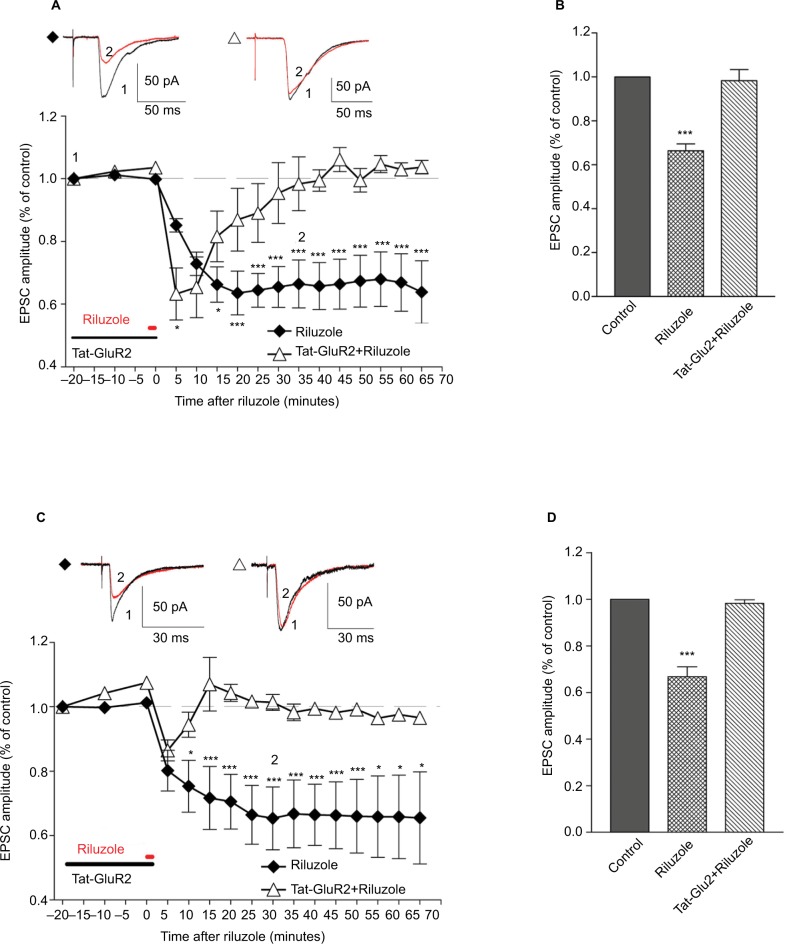
Figure 2.
Blocking of GluR2 endocytosis prevents riluzole-induced LTD of spinal nociceptive inputs.
Notes: Riluzole (50 µM)-induced LTD on C fiber (A) and A fiber (C) drive to neurons in SDH. The LTD was prevented by Tat-GluR2 (10 pmol/L). (B, D) Bar graphs showing that averaged peak values of eEPSCs are significantly reduced by 50 µM riluzole compared to those averaged from the control and Tat-GluR2 group. ***P<0.001, n=6, paired t-test, one-way ANOVA. *P<0.05.
Abbreviations: GluR2, glutamate receptor 2; LTD, long-term depression; eEPSC, evoked excitatory postsynaptic currents; ANOVA, analysis of variance; SDH, superficial dorsal horn.
Figure 3.
Riluzole decreases the frequency and amplitude of mEPSC.
Notes: (A) Ten successive episodes of mEPSCs recorded in ACSF. (B) Ten successive episodes of mEPSCs were recorded for riluzole (50 µM). All recordings were made during the application of TTX. (C) Comparison of cumulative probability of the inter-event interval of mEPSCs recorded in ACSF and riluzole. (D) Comparison of cumulative probability of amplitudes. HP = −70 mV. P<0.01, n=8, K–S test.
Abbreviations: mEPSC, miniature excitatory postsynaptic current; ACSF, artificial cerebrospinal fluid; TTX, tetrodotoxin.
Riluzole exerts a potent and long-lasting analgesic effect in models of pathological pain
It is well-accepted that a disruption of the balance between inhibitory and excitatory synaptic activities in the SDH contributes to neuropathic pain.4,15,22–24 To test the effect of riluzole on network hyperexcitability in pathological conditions, we created an in vitro disinhibition model in spinal slices by bath application of a mixture of bicuculline and strychnine – GABAA and Gly receptor antagonists. When HPs were set at −50 mV in current clamp mode, stimulation to the DR produced a biphasic response, polysynaptic inhibitory postsynaptic potentials (IPSPs), and monosynaptic C- fiber-mediated EPSPs in certain lamina II neurons (8/20, Figure 4A). The amplitudes of the EPSPs were relatively small, because they were masked by the IPSPs. The application of bicuculline (2 µM) and strychnine (20 µM) successfully blocked the IPSPs and generated long-lasting repetitive action potentials (6/6, Figure 4B). Using this mimicked pathological disinhibition model, we found that riluzole (50 µM) totally suppressed bicuculline- and strychnine-induced action potentials in all neurons tested (6/6, Figure 4C).
Figure 4.
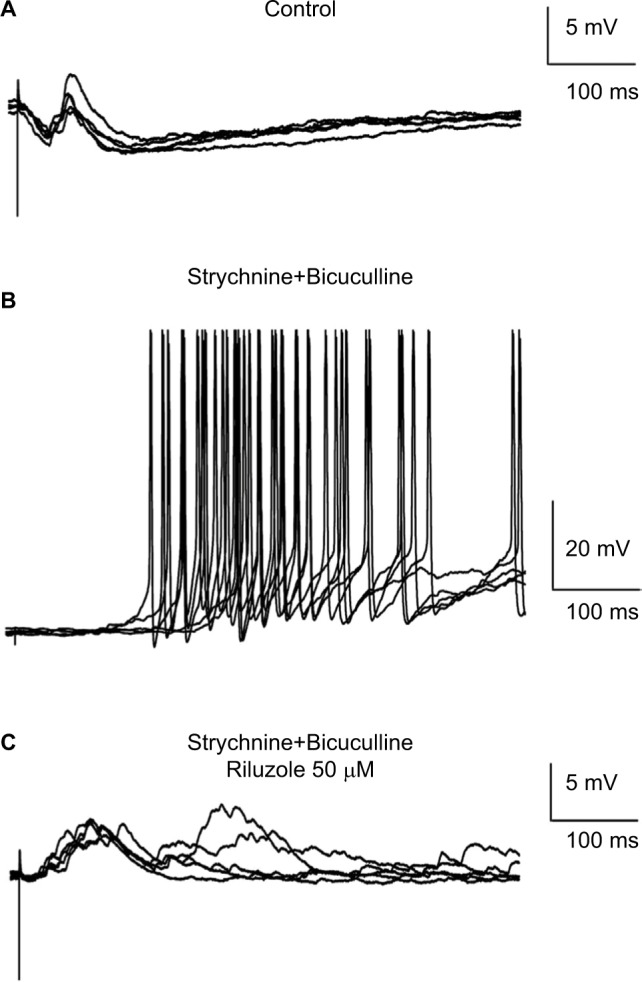
Riluzole reduces the hyperexcitability of SDH neurons in a slice model of disinhibition.
Notes: (A) DR stimulation at C-fiber intensity evoked a biphasic response in a lamina II neuron. (B) The application of bicuculline (2 µM) and strychnine (20 µM) successfully blocked the IPSPs and generated long-lasting repetitive action potentials. (C) Riluzole (50 µM) totally suppressed bicuculline- and strychnine-induced action potentials. HP was clamped at −50 mV in current clamp mode.
Abbreviations: SDH, superficial dorsal horn; DR, dorsal root; IPSP, inhibitory postsynaptic potential; HP, hold potential.
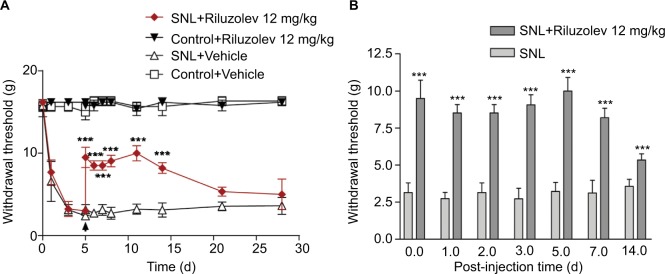
Using the well-established L5 SNL model, we next tested the actions of riluzole on nerve injury-induced pathological pain behaviors. Consistent with previous studies,6,13,25–29 we found that a single i.p. injection of riluzole (12 mg/kg) 5 days postoperatively produced pronounced and long-lasting anti-allodynia effects (P<0.001, n=6, Figure 5A). The 50% mechanical withdrawal thresholds were significantly increased as early as 3 hours post the riluzole injection. Notably, the analgesic effect persisted for at least 14 days (Figure 5B). These results, which were obtained both in vivo and in vitro, collectively suggested that riluzole had a potent and long-lasting analgesic effect in pathological conditions.
Figure 5.
A single intraperitoneal (i.p.) injection of riluzole produces long-lasting anti-allodynia effects.
Notes: (A) The lowered threshold of mechanical withdrawal indicated that nerve injury induces mechanical allodynia. The nerve injury-produced mechanical allodynia was partially reversed by i.p. injection of riluzole. (B) Bar graph shows the analgesic effect persisted for at least 14 days. ***P<0.01, one-way ANOVA with Bonferroni post hoc test, n=6.
Abbreviations: SNL, spinal nerve ligation; ANOVA, analysis of variance.
Postsynaptic AMPA receptors are critical for riluzole-induced postsynaptic inhibitory effects
Previous studies have indicated that riluzole’s neuroprotective effects may be associated with the inhibition of presynaptic Glu release in the central nervous system (CNS).5,6 Furthermore, the suppression of the mEPSC amplitude by riluzole indicated that riluzole produced inhibitory modulation of synaptic activity in postsynaptic levels in the spinal SDH. To further identify the putative receptors that mediated riluzole’s postsynaptic action in the presence of tetrodotoxin (TTX) (1 µM), the postsynaptic membrane currents induced by exogenous excitatory transmitters (Glu and AMPA) and inhibitory transmitters (GABA and Gly) were examined with or without riluzole. Compared with the control, riluzole suppressed the peak of inward currents induced by Glu and AMPA to 83.96±6.01 (P<0.05, n=5, Figure 6) and 77.69±3.01 (P<0.01, n=5, Figure 6), respectively, but had little effect on GABA- or Gly-induced currents (P>0.05, Figure 6). The above experiments suggested that riluzole could play a part in the postsynaptic inhibitory effect on certain SDH neurons through postsynaptic AMPA receptors.
Figure 6.
Riluzole decreases Glu- and AMPA-induced currents.
Notes: (A) Consecutive traces showing the membrane currents induced by Glu (100 µM), AMPA (100 µM), GABA (3 µM), and Gly (30 µM). Riluzole suppressed the peak of inward currents induced by Glu and AMPA, but had little effect on GABA- or Gly-induced currents (P>0.05, paired t-test, n=5). (B) The histogram indicates the comparison of membrane current peaks before and after dosing (mean ± SEM); *P<0.05, ***P<0.001, paired t-test, n=5).
Abbreviations: Glu, glutamic acid; AMPA, aminomethylphosphonic Acid; GABA, gamma-aminobutyric acid; Gly, glycine.
GluR2 endocytosis inhibitor prevents riluzole-induced LTD of C and Aδ fiber inputs
Because we found that riluzole inhibited excitatory synaptic transmission in the SDH in a concentration-dependent manner, we chose the concentration of 50 µM to investigate the long-lasting effects of riluzole. We found that 30-second perfusion of 50 µM riluzole suppressed C-fiber-evoked EPSCs to 66.41%±7.64% of control (P<0.001, n=6, Figure 2A and B), and Aδ fiber-evoked EPSCs to 66.75%±10.51% of control (P<0.001, n=6, Figure 2C and D) at 40 minutes post drug application. Notably, these suppression effects lasted throughout the entire observation period (>60 minutes), indicating an induction of LTD of C and Aδ fiber inputs.
It has been reported that endocytosis of AMPAR contributed to the induction of LTD in the CNS, and Tat-GluR2 was able to block the endocytosis of AMPAR in postsynaptic neurons.30–32 We tested whether endocytosis of GluR2 was involved in the riluzole-induced LTD. We found that preincubation of the slice with interference peptide of Tat-GluR2 (10 pmol/L, incubated for 20 minutes) prevented the LTD of C (P<0.001, n=6, Figure 2A, B) and Aδ (P<0.001, n=6, Figure 2C and D) fibers, supporting the hypothesis that the LTD caused by riluzole was associated with the endocytosis of GluR2 receptors.
To further identify that GluR2-mediated riluzole’s post-synaptic action, we carried out Western blotting tests. Results showed that the expression of membrane GluR2 significantly decreased to 54.8%±14.6% (P<0.05, one-way ANOVA, n=4), without a change in the vehicle group, at 3 days post riluzole treatment (i.p. 12 mg/kg; Figure 7A and B). The results indicated that riluzole could enhance the endocytosis of GluR2, a subtype of AMPAR, in postsynaptic neurons and induce LTD.
Figure 7.
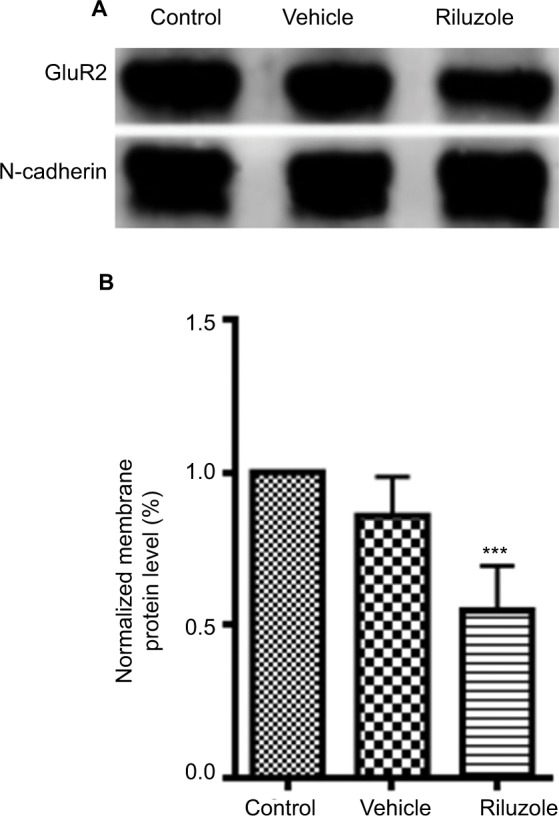
The expression of GluR2 receptor in membrane fraction was reduced by riluzole.
Notes: (A) Representative images of Western blotting bands. (B) Western blotting analysis demonstrated that riluzole (12 mg/kg intraperitoneal injection) in lumbar spinal cord reduced the expression of the GluR2 receptor in membrane fractions compared with control and vehicle groups (***P<0.001, one-way ANOVA, n=4, for each group).
Abbreviation: GluR2, glutamate receptor 2.
Discussion
Riluzole – a benzothiazole anticonvulsant – has long been used for treating ALS.33,34 Moreover, riluzole’s clinical applications include acute and chronic spinal cord injury,7,8 multiple sclerosis and Parkinson disease,9,10 and mental disorders.35,36 The underlying mechanism may be attributable to the regulation of the voltage-dependent sodium channel, enhanced activity of the small-conductance potassium channel, inhibition of calcium influx, modulation of Glu release, and blockade of NMDA receptor activation.13,37–39 Early reports demonstrated that riluzole had no analgesic action in a human model of neuropathic pain.40,41 However, increasing evidence has shown that riluzole has anti-nociceptive and anti-allodynic efficacies in rat models of SCI14 and in other animal models of neuropathic pain.6,13,25–29 Consistent with these studies, our data showed that systemic application of riluzole could produce a pronounced and long-lasting anti-allodynia effect in the maintenance phase of neuropathic pain. Most notably, our study is the first to show that the analgesic effect induced by a single injection of riluzole (12 mg/kg) strikingly persisted for at least 14 days, while its half-life is only 11.91±2.18 hours in spinal cord-injured patients.42
Increasing evidence indicates that a balance between inhibitory and excitatory synaptic activities in the SDH is disrupted by nerve injury, leading to the hyperexcitability of spinal sensory networks.4 In line with prior research,43,44 we used an in vitro pathological hyperexcitability model by bath application of a mixture of GABAA and Gly receptor antagonists. The application of strychnine and bicuculline resulted in hyperexcitability of SDH neurons through blocking the inhibitory transmitters. Using this pathological disinhibition model, we found that riluzole totally suppressed bicuculline- and strychnine-induced action potentials in SDH neurons, thereby reducing the hyperexcitability of the spinal nociceptive network.
Analyzing the amplitude and frequency of mEPSCs is a convenient method to distinguish the pre- and post- synaptic effects.45 The change of frequency indicates the presynaptic effect, whereas the change of amplitude indicates the post-synaptic effect. Riluzole significantly suppresses both the amplitude and frequency of mEPSCs, indicating that riluzole acts on both pre- and postsynaptic sites. Whereas many previous studies have reported presynaptic effects of riluzole,46,47 few studies have focused on their postsynaptic mechanisms. In light of this fact, our study focused on the postsynaptic mechanism of riluzole’s action. We further found that riluzole can significantly suppress the currents induced by Glu or AMPA, implying that the induction of LTD by riluzole was associated with AMPA receptors, whereas both NMDA and mGluR receptors mediating LTD eventually relied on the endocytosis of postsynaptic Glu AMPA receptors. This result is supported by the view that the downregulation of the AMPA receptors in the postsynaptic membrane contributes to the excitatory synaptic LTD in the CNS.48 It has been reported that riluzole administration reduces pain by decreasing the expression of pNR1 and pNR2B in astrocytes.13
In recent studies, TAT-GluR2 has been considered as a novel interfering peptide that interferes with the endocytosis of postsynaptic Glu AMPA receptors, which are responsible for the genesis of LTD.30–32 Based on the results of previous studies, we chose GluR2 to carry out our research. We found that Tat-GluR2 pretreatment reversed the later phase of riluzole-induced LTD but had little effect on the early phase. We, therefore, supposed that the reduction of Glu release might contribute to the early phase of riluzole’s action, whereas the later phase was induced by the promotion of the endocytosis of postsynaptic Glu AMPA receptors. Obviously, we cannot exclude the role of NMDA receptors and GluR1, 3, and 4 in the riluzole-induced LTD. This question deserves further study.
Conclusion
In conclusion, the present study finds that riluzole induces LTD of nociceptive signaling in the SDH and produces long-lasting anti-allodynia in nerve injury-induced neuropathic pain conditions via postsynaptic AMPA receptors associated with the endocytosis of GluR2. Our results suggest riluzole as a potential therapeutic approach for neuropathic pain.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos. 31530090 and 81471139 to YL; grant no. 81771183 to NG). The authors thank Christopher G. Myers (PhD, Johns Hopkins University) and Marybeth Myers (MD, Mt. Pleasant, SC) for language review of the manuscript.
Footnotes
Author contributions
Yandong Gao and Xiao Zhang conceived and designed the experiments. Xiao Zhang, Yandong Gao, Qun Wang, Shibin Du, and Xiaolan He carried out the experiments. Nan Gu and Yan Lu supervised the experiments and analyzed data. All authors contributed to data analysis, drafting and critical revision of the paper, gave final approval of the version to be submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978;177(3):417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- 2.Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186(2):133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- 3.Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234(4774):358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irifune M, Kikuchi N, Saida T, et al. Riluzole, a glutamate release inhibitor, induces loss of righting reflex, antinociception, and immobility in response to noxious stimulation in mice. Anesth Analg. 2007;104(6):1415–1421. doi: 10.1213/01.ane.0000263267.04198.36. [DOI] [PubMed] [Google Scholar]
- 6.Coderre TJ, Kumar N, Lefebvre CD, Yu JS. A comparison of the glutamate release inhibition and anti-allodynic effects of gabapentin, lamotrigine, and riluzole in a model of neuropathic pain. J Neurochem. 2007;100(5):1289–1299. doi: 10.1111/j.1471-4159.2006.04304.x. [DOI] [PubMed] [Google Scholar]
- 7.Fehlings MG, Nakashima H, Nagoshi N, Chow DS, Grossman RG, Kopjar B, Rationale KB. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016;54(1):8–15. doi: 10.1038/sc.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theiss RD, Hornby TG, Rymer WZ, Schmit BD. Riluzole decreases flexion withdrawal reflex but not voluntary ankle torque in human chronic spinal cord injury. J Neurophysiol. 2011;105(6):2781–2790. doi: 10.1152/jn.00570.2010. [DOI] [PubMed] [Google Scholar]
- 9.Bensimon G, Ludolph A, Agid Y, et al. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132(Pt 1):156–171. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nourbakhsh B, Revirajan N, Waubant E. Association Between Glutamate Blockade and Fatigue in Patients With Multiple Sclerosis. JAMA Neurol. 2015;72(11):1374–1375. doi: 10.1001/jamaneurol.2015.2332. [DOI] [PubMed] [Google Scholar]
- 11.Hayashida K, Parker RA, Eisenach JC. Activation of glutamate transporters in the locus coeruleus paradoxically activates descending inhibition in rats. Brain Res. 2010;1317:80–86. doi: 10.1016/j.brainres.2009.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie W, Strong JA, Kim D, Shahrestani S, Zhang JM. Bursting activity in myelinated sensory neurons plays a key role in pain behavior induced by localized inflammation of the rat sensory ganglion. Neuroscience. 2012;206:212–223. doi: 10.1016/j.neuroscience.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon ES, Karadimas SK, Yu WR, Austin JW, Fehlings MG. Riluzole attenuates neuropathic pain and enhances functional recovery in a rodent model of cervical spondylotic myelopathy. Neurobiol Dis. 2014;62:394–406. doi: 10.1016/j.nbd.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Hama A, Sagen J. Antinociceptive effect of riluzole in rats with neuropathic spinal cord injury pain. J Neurotrauma. 2011;28(1):127–134. doi: 10.1089/neu.2010.1539. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Dong H, Gao Y, et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest. 2013;123(9):4050–4062. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23(25):8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Perl ER. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J Neurophysiol. 1994;72(4):1611–1621. doi: 10.1152/jn.1994.72.4.1611. [DOI] [PubMed] [Google Scholar]
- 18.Gu N, Peng J, Murugan M, et al. Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell Rep. 2016;16(3):605–614. doi: 10.1016/j.celrep.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng J, Gu N, Zhou L, et al. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun. 2016;7:12029. doi: 10.1038/ncomms12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu N, Niu JY, Liu WT, et al. Hyperbaric oxygen therapy attenuates neuropathic hyperalgesia in rats and idiopathic trigeminal neuralgia in patients. Eur J Pain. 2012;16(8):1094–1105. doi: 10.1002/j.1532-2149.2012.00113.x. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II. J Neurosci. 2005;25(15):3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeilhofer HU. Loss of glycinergic and GABAergic inhibition in chronic pain--contributions of inflammation and microglia. Int Immunopharmacol. 2008;8(2):182–187. doi: 10.1016/j.intimp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Nickel FT, Seifert F, Lanz S, Maihöfner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;22(2):81–91. doi: 10.1016/j.euroneuro.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Chew DJ, Carlstedt T, Shortland PJ. The effects of minocycline or riluzole treatment on spinal root avulsion-induced pain in adult rats. J Pain. 2014;15(6):664–675. doi: 10.1016/j.jpain.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Jiang K, Zhuang Y, Yan M, et al. Effects of riluzole on P2X7R expression in the spinal cord in rat model of neuropathic pain. Neurosci Lett. 2016;618:127–133. doi: 10.1016/j.neulet.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 27.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23(7):2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson KJ, Zhang S, Gilliland TM, Winkelstein BA. Riluzole effects on behavioral sensitivity and the development of axonal damage and spinal modifications that occur after painful nerve root compression. J Neurosurg Spine. 2014;20(6):751–762. doi: 10.3171/2014.2.SPINE13672. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JM, Ji G, Neugebauer V. Small-conductance calcium-activated potassium (SK) channels in the amygdala mediate pain-inhibiting effects of clinically available riluzole in a rat model of arthritis pain. Mol Pain. 2015;11:51. doi: 10.1186/s12990-015-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, Kesner P, Metna-Laurent M, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148(5):1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11(7):459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 32.Wong TP, Howland JG, Robillard JM, et al. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci U S A. 2007;104(27):11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vucic S, Lin CS, Cheah BC, et al. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain. 2013;136(Pt 5):1361–1370. doi: 10.1093/brain/awt085. [DOI] [PubMed] [Google Scholar]
- 34.Bensimon G, Lacomblez L, Meininger V. A Controlled Trial of Riluzole in Amyotrophic Lateral Sclerosis. N Engl J Med Overseas Ed. 1994;330(9):585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 35.Pittenger C, Bloch MH, Wasylink S, et al. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a pilot randomized placebo-controlled trial. J Clin Psychiatry. 2015;76(8):1075–1084. doi: 10.4088/JCP.14m09123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutta A, Mckie S, Deakin JFW. Ketamine and other potential glutamate antidepressants. Psychiatry Res. 2015;225(1-2):1–13. doi: 10.1016/j.psychres.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 38.Ren SC, Chen PZ, Jiang HH, et al. Persistent sodium currents contribute to Aβ1-42-induced hyperexcitation of hippocampal CA1 pyramidal neurons. Neurosci Lett. 2014;580:62–67. doi: 10.1016/j.neulet.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 39.Theile JW, Cummins TR. Inhibition of Navβ4 peptide-mediated resurgent sodium currents in Nav1.7 channels by carbamazepine, riluzole, and anandamide. Mol Pharmacol. 2011;80(4):724–734. doi: 10.1124/mol.111.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammer NA, Lillesø J, Pedersen JL, Kehlet H. Effect of riluzole on acute pain and hyperalgesia in humans. Br J Anaesth. 1999;82(5):718–722. doi: 10.1093/bja/82.5.718. [DOI] [PubMed] [Google Scholar]
- 41.Galer BS, Twilling LL, Harle J, Cluff RS, Friedman E, Rowbotham MC. Lack of efficacy of riluzole in the treatment of peripheral neuropathic pain conditions. Neurology. 2000;55(7):971–975. doi: 10.1212/wnl.55.7.971. [DOI] [PubMed] [Google Scholar]
- 42.Chow DS, Teng Y, Toups EG, et al. Pharmacology of riluzole in acute spinal cord injury. J Neurosurg Spine. 2012;17(1 Suppl):129–140. doi: 10.3171/2012.5.AOSPINE12112. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol. 2007;582(Pt 1):127–136. doi: 10.1113/jphysiol.2007.131565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baba H, Ji RR, Kohno T, et al. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24(3):818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 45.Tian L, Ji G, Wang C, Bai X, Lu Y, Xiong L. Excitatory synaptic transmission in the spinal substantia gelatinosa is under an inhibitory tone of endogenous adenosine. Neurosci Lett. 2010;477(1):28–32. doi: 10.1016/j.neulet.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther. 2011;17(1):4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sámano C, Nasrabady SE, Nistri A. A study of the potential neuro-protective effect of riluzole on locomotor networks of the neonatal rat spinal cord in vitro damaged by excitotoxicity. Neuroscience. 2012;222:356–365. doi: 10.1016/j.neuroscience.2012.06.064. [DOI] [PubMed] [Google Scholar]
- 48.Kim YH, Back SK, Davies AJ, et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron. 2012;74(4):640–647. doi: 10.1016/j.neuron.2012.02.039. [DOI] [PubMed] [Google Scholar]