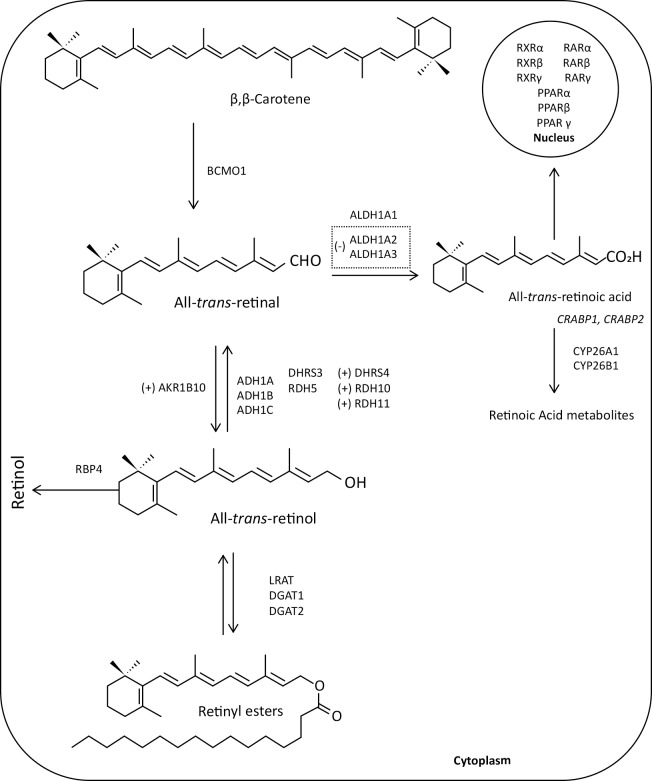
Fig 1. Intracellular metabolism of retinol (vitamin A) in the liver.
Retinol is provided by our diet and transported via chylomicrons into the liver, where it is bound to retinol binding protein (RBP). Retinol is converted to retinyl ester by lecithin retinol acyltransferase (LRAT), for storage. Retinol is converted into all-trans-retinal in the liver via two oxidative steps. The first enzymatic step is the reversible oxidation of retinol to retinal by three types of enzymes: i) members of retinal dehydrogenase family (RDH5, RDH10, RDH11), ii) alcohol dehydrogenase (ADH1, ADH1B and ADH1C); and iii) membrane-bound short-chain dehydrogenases/reductases (DHRS3 and DHRS4), all involved in maintaining an equilibrium between retinal and retinol. The second and irreversible step is the oxidation of retinal to all-trans-retinoic acid by retinaldehyde dehydrogenase 1 family, member A1, A2 and A3 (ALDH1A1, ALDH1A2 and ALDH1A3). Excessive all-trans-retinoic acid, which is bound to cellular retinoic acid-binding proteins (CRABP1 and CRABP2), is not recycled back to retinol and must be oxidized to be eliminated from the body by the cytochrome P450 family members CYP26A1 and CYP26B1. Newly synthesized all-trans-retinoic acid can be bound to CRABP2. All-trans-retinoic acid that enters the nucleus, binds to a retinoic acid receptor/retinoic X receptor (RAR/RXR) heterodimer and stimulates transcription of target genes. The plus sign (+) indicates increased expression of the enzyme AKR1B10, whereas the minus sign (-) indicates a reduced expression of the enzymes ALDH1A2 and ALDH1A3.