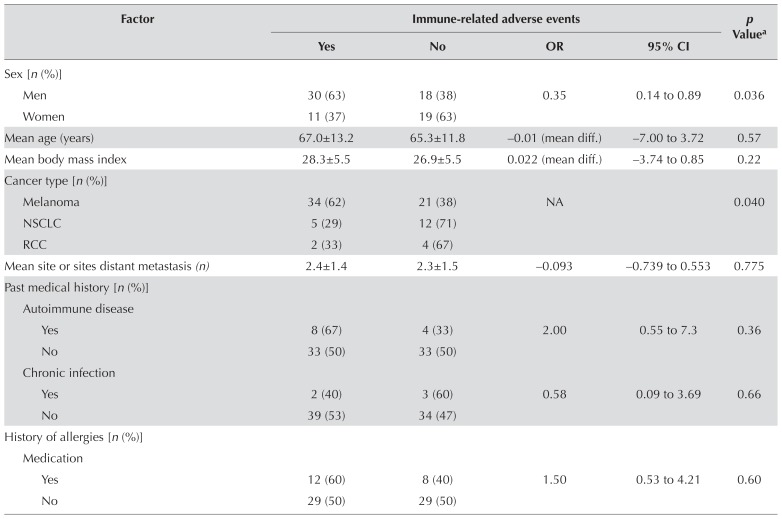
TABLE II.
Bivariate analyses of risk factors in relation to immune-related adverse events
| Factor | Immune-related adverse events | p Valuea | |||
|---|---|---|---|---|---|
|
| |||||
| Yes | No | OR | 95% CI | ||
| Sex [n (%)] | |||||
| Men | 30 (63) | 18 (38) | 0.35 | 0.14 to 0.89 | 0.036 |
| Women | 11 (37) | 19 (63) | |||
|
| |||||
| Mean age (years) | 67.0±13.2 | 65.3±11.8 | −0.01 (mean diff.) | −7.00 to 3.72 | 0.57 |
|
| |||||
| Mean body mass index | 28.3±5.5 | 26.9±5.5 | 0.022 (mean diff.) | −3.74 to 0.85 | 0.22 |
|
| |||||
| Cancer type [n (%)] | |||||
| Melanoma | 34 (62) | 21 (38) | NA | 0.040 | |
| NSCLC | 5 (29) | 12 (71) | |||
| RCC | 2 (33) | 4 (67) | |||
|
| |||||
| Mean site or sites distant metastasis (n) | 2.4±1.4 | 2.3±1.5 | −0.093 | −0.739 to 0.553 | 0.775 |
|
| |||||
| Past medical history [n (%)] | |||||
| Autoimmune disease | |||||
| Yes | 8 (67) | 4 (33) | 2.00 | 0.55 to 7.3 | 0.36 |
| No | 33 (50) | 33 (50) | |||
| Chronic infection | |||||
| Yes | 2 (40) | 3 (60) | 0.58 | 0.09 to 3.69 | 0.66 |
| No | 39 (53) | 34 (47) | |||
|
| |||||
| History of allergies [n(%)] | |||||
| Medication | |||||
| Yes | 12 (60) | 8 (40) | 1.50 | 0.53 to 4.21 | 0.60 |
| No | 29 (50) | 29 (50) | |||
|
| |||||
| History of allergies [n (%)] continued | |||||
| Environmental substances | |||||
| Yes | 4 (67) | 2 (33) | 1.89 | 0.33 to 11.0 | 0.68 |
| No | 37 (51) | 35 (49) | |||
|
| |||||
| Medication use [n (%)] | |||||
| Anti-arrhythmics | |||||
| Yes | 13 (65) | 7 (35) | 1.99 | 0.69 to 5.71 | 0.30 |
| No | 28 (48) | 30 (52) | |||
| Antihypertensives | |||||
| Yes | 21 (57) | 16 (43) | 1.38 | 0.56 to 3.37 | 0.51 |
| No | 20 (49) | 21 (51) | |||
| Antipsychotics | |||||
| Yes | 2 (67) | 1 (33) | 1.85 | 0.16 to 21.2 | 1.00 |
| No | 39 (52) | 36 (48) | |||
| Anticonvulsants | |||||
| Yes | 2 (40) | 3 (60) | 0.58 | 0.09 to 3.69 | 0.66 |
| No | 39 (53) | 34 (47) | |||
| Statins | |||||
| Yes | 22 (69) | 10 (31) | 3.13 | 1.21 to 8.09 | 0.022 |
| No | 19 (41) | 27 (59) | |||
| Steroids | |||||
| Yes | 7 (29) | 17 (71) | 0.24 | 0.09 to 0.69 | 0.007 |
| No | 34 (63) | 20 (37) | |||
| Allopurinol | |||||
| Yes | 2 (100) | 0 (0) | NA | 0.50 | |
| No | 39 (51) | 37 (49) | |||
| NSAIDs | |||||
| Yes | 5 (56) | 4 (44) | 1.15 | 0.28 to 4.63 | 1.00 |
| No | 36 (52) | 33 (48) | |||
| Salicylates | |||||
| Yes | 7 (70) | 3 (30) | 2.33 | 0.56 to 9.79 | 0.32 |
| No | 34 (50) | 34 (50) | |||
| Metformin | |||||
| Yes | 5 (56) | 4 (44) | 1.15 | 0.28 to 4.63 | 1.00 |
| No | 36 (52) | 33 (49) | |||
|
| |||||
| Baseline eGFRb [n (%)] | |||||
| Grades 1–2 | 29 (46) | 34 (54) | 4.70 | 1.21 to 18.2 | 0.022 |
| Grades 3–4 | 12 (80) | 3 (20) | |||
|
| |||||
| Immunotherapy agent [n (%)] | |||||
| Ipilimumab | 17 (68) | 8 (32) | NA | 0.054 | |
| Pembrolizumab | 15 (56) | 12 (44) | |||
| Nivolumab | 9 (35) | 17 (65) | |||
|
| |||||
| Overlap of RT and immunotherapy [n (%)] | |||||
| Yes | 24 (65) | 13 (35) | 2.61 | 1.04 to 6.52 | 0.045 |
| No | 17 (42) | 24 (59) | |||
| Mean days of overlap | 5.0±13.7 | 1.5±2.4 | −3.48 (mean diff.) | −9.61 to 2.66 | 0.26 |
Two-sided.
As defined by The Renal Association (Bristol, U.K.): grade 1, ≥90 mL/min/1.73 m2; grade 2, 60–89.9 mL/min/1.73 m2; grade 3a, 45–59.9 mL/min/1.73 m2; grade 3b, 30–44.9 mL/min/1.73 m2; grade 4, 15–29.9 mL/min/1.73 m2.
OR = odds ratio; CI = confidence interval; NSCLC = non-small-cell lung cancer; RCC = renal cell carcinoma; NSAIDs = nonsteroidal anti-inflammatory drugs; eGFR - estimated glomerular filtration rate; RT = radiotherapy.