Abstract
AIM
To detect the mechanisms of Helicobacter pylori (H. pylori) infection in the invasion and metastasis of gastric cancer (GC).
METHODS
Specimens from 99 patients with GC were collected. The correlation among H. pylori infection, heparanase (HPA) and mitogen-activated protein kinase (MAPK) expression, which was determined by immunohistochemistry, and the clinical features of GC was analysed using SPSS 22.0. Overall survival (OS) and relapse-free survival (RFS) of GC patients were estimated by the Kaplan-Meier method. Independent and multiple factors of HPA and MAPK with prognosis were determined with COX proportional hazards models. HPA and MAPK expression in MKN-45 cells infected with H. pylori was analysed using Western blot.
RESULTS
H. pylori infection was observed in 70 of 99 patients with GC (70.7%), which was significantly higher than that in healthy controls. H. pylori infection was related to lymph metastasis and expression of HPA and MAPK (P < 0.05); HPA expression was relevant to MAPK expression (P = 0.024). HPA and MAPK expression in MKN-45 cells was significantly upregulated following H. pylori infection and peaked at 24 h and 60 min, before decreasing (P < 0.05). SB203580, an inhibitor of MAPK, significantly decreased HPA expression. HPA was related to lymph metastasis and invasive depth. HPA positive GC cases and H. pylori positive GC cases showed poorer prognosis than HPA negative cases (P < 0.05). COX models showed that the prognosis of GC was connected with HPA expression, lymph metastasis, tissue differentiation, and invasive depth.
CONCLUSION
H. pylori may promote the invasion and metastasis of GC by increasing HPA expression that may associate with MAPK activation, thus causing a poorer prognosis of GC.
Keywords: Gastric cancer, Helicobacter pylori, Heparanase, Mitogen-activated protein kinase, Overall survival, Relapse-free survival
Core tip: The mechanism of Helicobacter pylori (H. pylori) infection in the invasion and metastasis of gastric cancer (GC) is still unknown. This paper studied heparanase (HPA) and mitogen-activated protein kinase (MAPK) expression in GC tissues and GC cells and their relationship with H. pylori infection. H. pylori infection may promote the invasion and metastasis of GC by increasing the expression of HPA that may be increased by activation of MAPK signal and HPA expression in GC tissue. H. pylori positive GC had a poorer prognosis.
INTRODUCTION
Helicobacter pylori (H. pylori) has been classified as a class I carcinogen by the International Agency for Research on Cancer[1]. H. pylori can live in the acidic environment of the stomach for a long time, and its prolonged infection can destroy the gastric mucosa and result in changes in the release of gastric mucosal hormones, thus affecting the physiological state of the stomach. Therefore, H. pylori infection represents the most significant risk factor for malignant gastric tumours[2,3]. Approximately 50% of the world’s population are infected with H. pylori[4,5]. The infection rate in China may be as high as 73.3%[6], especially in the Beijing region, where the infection rate is as high as 83.4%[7]. Although there have been increasing numbers of studies indicating that H. pylori infection can result in gastric cancer (GC), the underlying mechanism is still unknown.
Heparanase (HPA) is an endoglycosidase that is capable of degrading heparan sulfate (HS) in the extracellular matrix (ECM) and basement membrane (BM)[8,9], the process of which releases many types of biological mediators, such as fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF), in response to local or systemic signals[10,11]. Thus, HPA is involved in tissue remodelling and cell migration, which lead to inflammation, angiogenesis, and tumour metastasis[12-15]. The degradation of the ECM is one of the key steps involved in the invasion and metastasis of malignant tumours, and matrix degradation primarily depends on proteolytic enzymes. An increasing number of studies have demonstrated that the invasion and metastasis of tumour cells are closely associated with HPA production[16-18], including those derived from the stomach, pancreas, colon, and bladder. In addition to its enzymatic activity, recent studies have shown that the non-enzyme activity of HPA promotes the aggregation of heparan sulfate proteoglycans (HSPGs), causing a cascade of intracellular signal amplification that results in the activation of protein kinase C (PKC), Src, and Rac. HSPGs act on HPA receptors located on the cell surface, such as 6-phosphate mannose receptor (MPR), cationic non-6-phosphate-dependent mannose receptor (CD222), and low-density lipoprotein receptor-related protein (LRP), to cause signalling cascades. In addition, HPA plays an important role in inflammation and autoimmune diseases (e.g., colitis, arthritis, psoriasis, and sepsis)[19,20].
Some studies have shown that H. pylori infection leads the development of gastric adenocarcinoma by activating mitogen-activated protein kinase (MAPK)[21,22]. Once activated, MAPK is translocated to the nucleus and leads to the activation of transcription factors, such as NF-κB[23,24]. A recent study[25] has also shown that the activation of the MAPK pathway is closely related to the expression of HPA. However, it is not clear whether MAPK is involved in the regulation of HPA expression following H. pylori infections that lead to GC.
The present research aimed to explore the role of H. pylori infection in GC and analyse the connections among H. pylori infection, HPA and MAPK expression, and the pathological state of GC. By detecting the expression of HPA and MAPK and H. pylori in GC tissue and the expression of HPA and MAPK in MKN-45 cells infected by H. pylori, we analysed their associations with the clinical and pathological features of GC, and the survival and prognosis of H. pylori or HPA/MAPK positive GC.
MATERIALS AND METHODS
Cells and H. pylori strain
MKN-45 (human GC cell line) cells were obtained from the Chinese Academy of Sciences (Shanghai, China), preserved in the Key Laboratory of Digestive System Tumours of the Second Clinical Medical School of Lanzhou University, and cultivated in RPMI-1640 (HyClone Laboratories, Inc., Logan, UT, United States) supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc.), 100 U/mL penicillin, and 100 mg/mL streptomycin (North China Pharmaceutical Co., Inc., Shijiazhuang, China) in humidified air containing 5% carbon dioxide at 37 °C.
H. pylori NCTC11637 was provided by the Key Institute of Digestion and Oncology of Gansu Province and was cultured on Columbia agar plates (Solarbio, Shanghai, China) containing 7% filtered goat blood in an anaerobic tank.
Infection of MKN-45 cells with H. pylori
After digestion, MKN-45 cells were inoculated in three culture dishes with an equal cell volume and cultured under normal growth conditions until they reached the logarithmic growth phase, at which point the old medium was discarded and replaced with culture medium containing serum and antibiotics. H. pylori was cultured at 37 °C for 72 h under microaerophilic conditions using an anaerobic box. H. pylori was then added to the above cell medium at a bacteria:cell ratio of 100:1. The bacteria and cells were co-cultured for 6, 12, 24, and 48 h at 37 °C in 5% CO2 and saturated humidity to detect HPA and MAPK expression.
Western blot assay
Total protein of the treated cells was extracted using RIPA lysis buffer (Beyotime Biotechnology, Haimen, China), phenylmethanesulfonyl fluoride (PMSF) (Beyotime Biotechnology), and a protein phosphatase inhibitor (Solarbio Biotechnology Co., Shanghai, China) after being washed with ice-cold phosphate-buffered saline (PBS). The protein concentration was measured by the BCA protein assay (Beyotime Biotechnology) after centrifugation at 14000 rpm for 30 min. The protein was separated on a 10% SDS polyacrylamide gel and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Solarbio Biotechnology), which was then blocked in the blocking solution for 2 h at a constant temperature. Then, the above membrane was incubated with the following primary antibodies: anti-heparanase1 (Abcam Biotechnology, Cambridge, United Kingdom), anti-phospho-p38, anti-p38 (Abcam Biotechnology), and anti-β-actin (Zhongshan Golden Bridge Biotech, Beijing, China). After overnight incubation, the membrane was washed three times with Tris Buffered Saline-Tween (TBST) (Solarbio Biotechnology) and then incubated with a horseradish peroxidase-conjugated secondary antibody (Zhongshan Golden Bridge Biotech; dilution 1:10000) at room temperature for 1 h. The SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific, Inc., Rockford, IL, United States) was used to detect signals, which were displayed with a VersaDoc Imaging System (Bio-Rad Laboratories Co., Ltd. Hercules, CA, United States). Data were analysed using Bio-Rad Quantity One Software v4.62 (Bio-Rad Laboratories Co., Ltd.). To further illustrate whether the H. pylori-induced upregulation of HPA was mediated through the MAPK pathway, 20 μmol/L of the MAPK inhibitor SB203580 (Selleck, United States) was added to MKN-45 cells for 2 h before they were cultured with H. pylori.
Transwell assay
A Transwell assay was used to detect the invasion capability of the tumour cells. A 1:8 mixture of Matrigel gel:RPMI-1640 medium (100 μL) was added to the lower chamber, which was placed in 24-well plates at 37 °C for 24 h. A serum-free cell suspension (200 μL) at a concentration of 2.5 × 105 cells/mL was added to the upper chamber, while serum-containing medium was added to the lower chamber. Then, the cells were cultured at 37 °C in an atmosphere with 5% CO2 and saturated humidity for 24 h. The remaining cells in the upper chamber were carefully wiped off, and the lower chamber was rinsed twice with PBS and fixed in methanol for 15 min. The cells were stained with crystal violet solution in methanol for 30 min, and then excess crystal violet was washed off. The cells were observed and images were obtained using a microscope. The number of migrated cells was counted in several fields of view.
Scratch test
The scratch test was used to detect the migration ability of tumour cells. Horizontal lines were drawn every 0.5-1 cm on the back of a 6-well plate and 5 × 105 cells were added to each well. The cells were incubated overnight and when cells were 100% confluent, a cut was made across the dish with a 200-mL pipette. The cells were rinsed twice with PBS, serum-free medium was added, and the cells were incubated at 37 °C in an atmosphere with 5% CO2. Following the incubation, images of the cells were obtained and the migration distance was measured.
Patients and clinicopathological characteristics
Ninety-nine cases of pathologically diagnosed and surgically resected primary gastric carcinoma tissues and tumour-adjacent tissues (> 5 cm from the edge of the neoplastic foci and non-tumour tissue confirmed by pathology) without preoperative chemotherapy or radiotherapy from the Department of Gastroenterological and Oncological Surgery of the First Hospital of Lanzhou University were collected between June 2013 and June 2014. Patients with GC were aged between 31 and 77 years, with a mean age of 59.5 ± 9.8 years, and included 61 males and 38 females. Of the 99 cases of GC, there were 48 cases of highly and moderately differentiated carcinoma, 51 cases of poorly differentiated carcinoma, 55 cases of lymph node metastasis, and 44 cases without lymph node metastasis. With respect to the extent of infiltration, there were 42 cases of the T1/2 stage and 57 cases of the T3/4 stage. The clinicopathological characteristics are shown in Table 1. All cases of GC had received D2 radical surgery, and paraffin sections of GC tissue were taken from the Department of Pathology, First Hospital of Lanzhou University. The patient’s clinical data, pathological results, and follow-up data were all recorded in detail. Except for stage Ia patients with GC, patients were given postoperative chemotherapy regimens of 6 cycles of XELOX. A total of 25 healthy controls were selected from the Department of Gastroenterology, who required gastroscopy to exclude digestive system tumours and diseases, including 16 males and 9 females, with an average age of 40 years. The follow-up of patients with GC was performed by a specialist. Overall survival time was measured from the date of surgery to the date of death due to any cause. All postoperative cases were followed for 3-60 mo. All research complied with the “Methods for Ethical Review of Biomedical Research Involving Human Beings (Trial)” and the Declaration of Helsinki. Prior written informed consent was obtained from every subject, and the study was approved by the Ethics Review Board of the First Hospital of Lanzhou University.
Table 1.
Heparanase and mitogen-activated protein kinase expression in specimens of gastric cancer n (%)
| Cases (n) |
Immunohistochemical result |
HR (P value) | ||||
| - | + | ++ | +++ | |||
| HPA | ||||||
| Gastric cancer | 99 | 30 (30.3) | 16 (16.2) | 25 (25.3) | 28 (28.3) | 35.547 (0.000) |
| Para-cancer | 99 | 57 (57.6) | 11 (11.1) | 17 (17.2) | 16 (16.2)b | |
| Normal tissue | 25 | 24 (96.0) | 1 (4.0) | 0 (0.0) | 0 (0.0)bd | |
| MAPK | ||||||
| Gastric cancer | 99 | 49 (49.5) | 12 (12.1) | 19 (19.2) | 19 (19.2) | 33.303 (0.000) |
| Para-cancer | 99 | 82 (82.8) | 5 (5.1) | 6 (6.1) | 6 (6.1)b | |
| Normal tissue | 25 | 23 (92.0) | 2 (8.0) | 0 (0.0) | 0 (0.0)b | |
P values < 0.05 were determined using one way analysis of variance (ANOVA):
P: Comparison with gastric cancer;
P: Comparison with para-cancer tissue. HPA: Heparanase; MAPK: Mitogen-activated protein kinase; HR: Hazard ratio.
Immunohistochemistry
Protein expression of HPA and MAPK was detected by immunohistochemical staining (the SP method) in GC and para-carcinoma tissues. Formaldehyde fixed and paraffin embedded sections of samples were dewaxed, rehydrated with different concentrations of alcohol, and prepared for antigen retrieval with citrate by the high-temperature and high-pressure method. Primary antibodies were added to the sections and incubated at 37 °C in the dark for 1 h. Then, secondary antibodies were added for 30 min. Next, DAB chromogenic reagent was added to develop and hematoxylin was added to stain. The working concentrations of the primary antibodies against HPA and MAPK were both 1:200, and the negative control group used PBS instead of the primary antibody. Positive staining for HPA and MAPK was both primarily located in the cytoplasm. The semi-quantitative scoring criteria were as follows: according to the percentage of positive cells and staining intensity of each slice, the positive staining cell ratio was recorded as 0 points, 1 point, 2 points and 3 points for < 5%, 5% to 25%, 26% to 50%, and > 50%, respectively; the staining intensity was defined as 0 points, 1 point, 2 points and 3 points for no staining, pale yellow, brownish yellow and tan, respectively.
H. pylori infection status
To identify the infection status of H. pylori in GC and adjacent tissues, immunohistochemistry was used to test for H. pylori infection with a rabbit polyclonal anti-H. pylori antibody (DAKO). All diagnosed GC patients underwent a C13 breath test before surgery to check for H. pylori infection. If the clinical C13 exhalation test and immunohistochemical staining of the surgical pathology section were both confirmed to be positive for H. pylori, the patient was defined as H. pylori positive.
Statistical analysis
Data were analysed using SPSS 22.0 statistical software (IBM, Amonk, NY, United States). The classified data are described as the number of cases and rate or constituent ratio (%). The Chi-square test was used to compare classified disordered data groups. The Kruskal-Wallis H test was used to compare the classified ordered data. Association analysis between H. pylori infection and the expression of HPA and MAPK was analysed by the chi-square test, and the contingency coefficient was calculated. The survival rates of the different groups were analysed by the Kaplan-Meier method and log-rank (mantel-COX) test. Univariate and multivariate COX regression analyses were used to explore the influencing factors of the survival time. P < 0.05 was considered statistically significant.
RESULTS
HPA and MAPK protein expression in GC tissue is higher than that in para-carcinoma tissue and normal gastric tissue
To detect HPA and MAPK protein expression in GC, immunohistochemical staining was carried out. Representative results from the immunohistochemical staining are shown in Figure 1 and detailed data are shown in Table 1. As shown in Figure 1, HPA and MAPK were not basally expressed in normal gastric tissue and were seldom expressed in para-carcinoma tissue; however, expression of HPA and MAPK was significantly positive in GC tissue. As seen in Table 1, HPA and MAPK were not basally expressed in normal gastric tissue, but expression of HPA and MAPK was positive in GC and para-carcinoma tissues, and expression of HPA and MAPK in GC tissue was significantly higher than that in para-carcinoma and normal tissues.
Figure 1.

Immunohistochemical analysis of heparanase and mitogen-activated protein kinase protein expression in gastric cancer. Expression of heparanase (HPA) and mitogen-activated protein kinase (MAPK) was detected by immunohistochemical staining in normal gastric tissue. Representative immunohistochemical staining images are shown (magnification, 200 ×). HPA: Heparanase; MAPK: Mitogen-activated protein kinase.
H. pylori infection in GC is relevant to the expression of HPA and MAPK and leads to higher expression than that in normal gastric tissue
To detect the H. pylori infection levels, the clinical C13 exhalation test and immunohistochemical staining of surgical sections were used. There were 70 cases of H. pylori infection out of 99 GC cases, with a positive rate of 70.71%, and 10 cases of H. pylori infection out of 25 healthy control cases, with a positive rate of 40.0%. There was a significant difference between the levels of H. pylori infection in GC and those of normal gastric tissue (P < 0.05), as revealed in Table 2. This result suggests that there is a significant correlation between H. pylori infection and GC.
Table 2.
Correlation between Helicobacter pylori infection and gastric cancer n (%)
| H. pylori infection | Gastric cancer (n = 99) | Normal tissue (n = 25) | χ2 | P value | C |
| Negative | 29 (29.29) | 15 (60.00) | 8.221 | 0.004 | 0.229 |
| Positive | 70 (70.71) | 10 (40.00) |
H. pylori: Helicobacter pylori.
Correlation between H. pylori infection, expression of HPA and MAPK proteins, and pathological characteristics of GC
To ascertain the effects of H. pylori infection and HPA and MAPK protein expression in GC, the associations among the clinical pathological characteristics of gastric cancer and the above factors were analysed. Positive H. pylori infection and positive expression of HPA were both associated with lymph node metastasis (P < 0.05), but not with age, gender, diameter of tumour, or differentiation degree (P > 0.05). Similarly, positive HPA expression and positive MAPK expression were both associated with the depth of invasion (P < 0.05), as shown in Table 3.
Table 3.
Correlation analyses of Helicobacter pylori infection, heparanase and mitogen-activated protein kinase expression, and gastric cancer pathological characteristics n (%)
| Parameter | Cases (n) |
H. pylori infection |
P value |
HPA expression |
P value |
MAPK expression |
P value | |||
| Positive | Negative | Positive | Negative | Positive | Negative | |||||
| Age (yr) | ||||||||||
| < 60 | 46 | 32 (32.3) | 14 (14.1) | 0.816 | 31 (31.3) | 14 (14.1) | 0.8731 | 22 (22.2) | 24 (24.2) | 0.619 |
| ≥ 60 | 53 | 38 (38.4) | 15 (15.2) | 38 (38.4) | 16 (16.2) | 28 (28.3) | 25 (25.3) | |||
| Gender | ||||||||||
| Male | 61 | 46 (46.5) | 15 (15.2) | 0.193 | 42 (42.4) | 18 (18.2) | 0.935 | 27 (27.3) | 34 (34.3) | 0.115 |
| Female | 38 | 24 (24.2) | 14 (14.1) | 27 (27.3) | 12 (12.1) | 23 (23.2) | 15 (15.2) | |||
| Lymph node metastasis | ||||||||||
| Without | 42 | 24 (24.2) | 18 (18.2) | 0.011 | 23 (23.2) | 19 (19.2) | 0.006 | 24 (24.2) | 18 (18.2) | 0.257 |
| With | 57 | 46 (46.5) | 11 (11.1) | 46 (46.5) | 11 (11.1) | 26 (26.3) | 31 (31.3) | |||
| Tumour diameter | ||||||||||
| < 40 mm | 57 | 40 (40.4) | 17 (17.2) | 0.892 | 39 (39.4) | 17 (17.2) | 0.989 | 25 (25.3) | 32 (32.3) | 0.123 |
| ≥ 40 mm | 42 | 30 (30.3) | 12 (12.1) | 30 (30.3) | 13 (13.1) | 25 (25.3) | 17 (17.2) | |||
| Differentiation degree | ||||||||||
| Poorly | 40 | 28 (28.3) | 12 (12.1) | 0.899 | 26 (26.3) | 14 (14.1) | 0.402 | 23 (23.2) | 17 (17.2) | 0.252 |
| Highly and moderately | 59 | 42 (42.4) | 17 (17.2) | 43 (43.3) | 16 (16.2) | 27 (27.3) | 32 (32.3) | |||
| Invasive depth | ||||||||||
| T1/2 | 39 | 31 (31.3) | 8 (8.1) | 0.122 | 33 (33.3) | 6 (6.1) | 0.009 | 24 (24.2) | 15 (15.2) | 0.077 |
| T3/4 | 60 | 39 (39.4) | 21 (21.2) | 36 (36.4) | 24 (24.2) | 26 (26.3) | 34 (34.3) | |||
HPA: Heparanase; MAPK: Mitogen-activated protein kinase; H. pylori: Helicobacter pylori.
There were 54 cases of HPA positive expression in 70 H. pylori positive GC cases, with a positive rate of 54.5%, and 15 cases of HPA positive expression in 29 H. pylori negative GC cases, with a positive rate of 15.2%. Obviously, significantly higher levels of HPA positive expression in H. pylori positive GC cases were observed than those in H. pylori negative GC cases (P < 0.05), as shown in Table 4. This result suggests that there is significant HPA expression in GC with H. pylori infection.
Table 4.
Correlation between heparanase expression and Helicobacter pylori infection in gastric cancer
| H. pylori infection |
HPA expression |
χ2 | P value | C | |
| Negative | Positive | ||||
| Negative | 14 (14.1) | 15 (15.2) | 6.273 | 0.012 | 0.244 |
| Positive | 16 (16.2) | 54 (54.5) | |||
HPA: Heparanase; H. pylori: Helicobacter pylori.
There were 40 cases with MAPK positive expression in 70 H. pylori positive GC cases, with a positive rate of 40.4%, and 10 cases of MAPK positive expression in 29 H. pylori negative GC cases, with a positive rate of 10.1%. Significantly higher levels of MAPK positive expression in H. pylori positive GC cases were observed than those in H. pylori negative GC cases (P < 0.05), as shown in Table 5.
Table 5.
Correlation between mitogen-activated protein kinase expression and Helicobacter pylori infection in gastric cancer
| H. pylori infection |
MAPK expression |
χ2 | P value | C | |
| Negative | Positive | ||||
| Negative | 19 (19.2) | 10 (10.1) | 4.212 | 0.04 | 0.202 |
| Positive | 30 (30.3) | 40 (40.4) | |||
MAPK: Mitogen-activated protein kinase; H. pylori: Helicobacter pylori.
There were 40 cases of MAPK positive expression in 69 HPA positive GC cases, with a positive rate of 39.4%, and 10 cases of MAPK positive expression in 30 HPA negative GC cases, with a positive rate of 11.1%. Significantly higher MAPK positive expression in HPA positive GC cases were observed than those in HPA negative GC cases (P < 0.05), as shown in Table 6. This result suggests that there is a significant correlation between HPA expression and MAPK expression in GC.
Table 6.
Correlation between heparanase expression and mitogen-activated protein kinase expression in gastric cancer
| HPA expression |
MAPK expression |
χ2 | P value | C | |
| Negative | Positive | ||||
| Negative | 20 (19.2) | 10 (11.1) | 5.077 | 0.024 | 0.221 |
| Positive | 29 (30.3) | 40 (39.4) | |||
HPA: Heparanase; MAPK: Mitogen-activated protein kinase.
H. pylori mediates the increase of HPA expression in MKN-45 cells via the MAPK signalling pathway
To detect the effect of H. pylori infection on HPA expression in GC cells, H. pylori and MKN-45 were co-cultured at a ratio of 100 bacteria: 1 cell for 0 h, 6 h, 12 h, 24 h, and 48 h. Western blot assay confirmed the enhancement of HPA expression at the protein level, and this level peaked at 24 h in H. pylori-infected GC cells (Figure 2A and B). To illustrate whether MAPK signalling is involved in H. pylori-induced expression of HPA, the expression of phosphorylated p38 MAPK (p-p38MAPK) was detected by Western blot analysis when H. pylori and MKN-45 were co-cultured for 0 min, 30 min, 60 min, 120 min, and 480 min. The expression of p-p38MAPK was significantly higher after 30 min and peaked at 60 min, whereas the total amount of p38MAPK remained unchanged (Figure 2C and D). To further illustrate whether H. pylori-induced upregulation of HPA is mediated through the MAPK pathway, 20 μmol/L of the MAPK inhibitor SB203580 was added to MKN-45 cells for 2 h before they were cultured with H. pylori. The expression of HPA protein was significantly higher when H. pylori infected MKN-45 cells, but that upregulation was significantly inhibited by SB203580 (Figure 2E and F). The Transwell invasion (Figure 2G and H) and scratch test migration (Figure 2I and J) assays confirmed that the addition of SB203580 to H. pylori-infected MKN-45 cells markedly decreased the invasion and migration abilities of MKN-45 cells.
Figure 2.
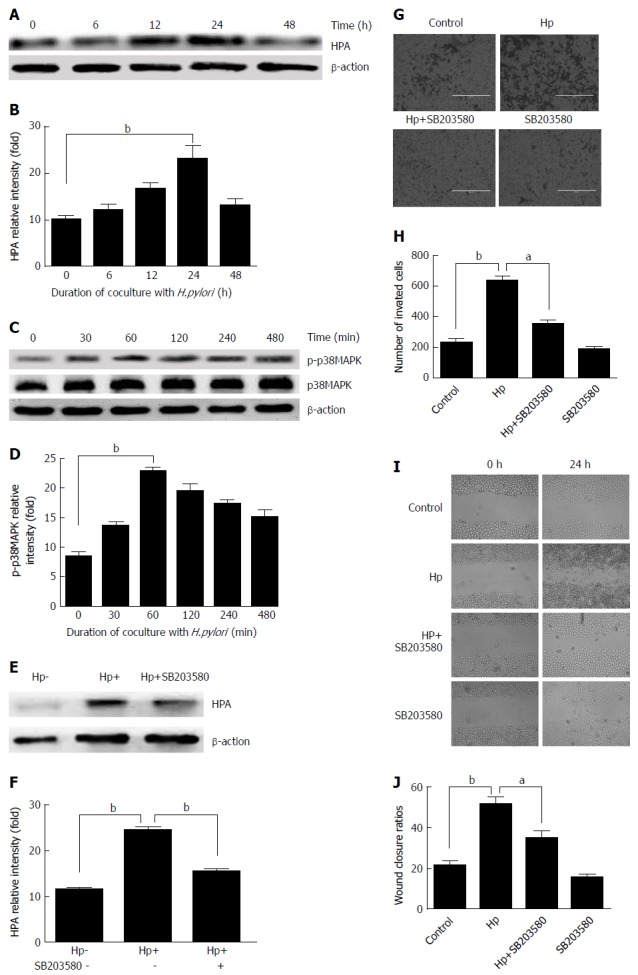
Heparanase protein expression following Helicobacter pylori infection in MKN-45 gastric cancer cells via the mitogen-activated protein kinase signaling pathway. A: Heparanase (HPA) expression was determined by Western blot at 0, 6, 12, 24, and 48 h after Helicobacter pylori (H. pylori) infection; B: Quantitative Western blot results of HPA; C: p-p38MAPK expression was determined by Western blot at 0, 30, 60, 120, and 480 min after H. pylori infection; D: Quantitative Western blot results of p-p38MAPK; E: HPA expression when the MAPK inhibitor SB203580 was given to MKN-45 cells before H. pylori infection; F: Quantitative Western blot results of HPA when the MAPK inhibitor SB203580 was given. bP < 0.01 compared with the value at 0 h. G, H: Cell invasion rates in the three groups detected using a Transwell invasion assay. I, J: Migration rates in the three groups detected using a scratch migration assay. aP < 0.05, bP < 0.01. HPA: Heparanase; MAPK: Mitogen-activated protein kinase; H. pylori: Helicobacter pylori.
H. pylori infection, HPA, and prognosis
HPA positive expression in GC significantly predicted poor overall survival (P = 0.000) and poor relapse-free survival (P = 0.006). Especially in H. pylori-infected GC, HPA positive expression was a more significant factor for predicting poor prognosis (overall survival, P = 0.000; relapse-free survival, P = 0.007) (Figure 3).
Figure 3.
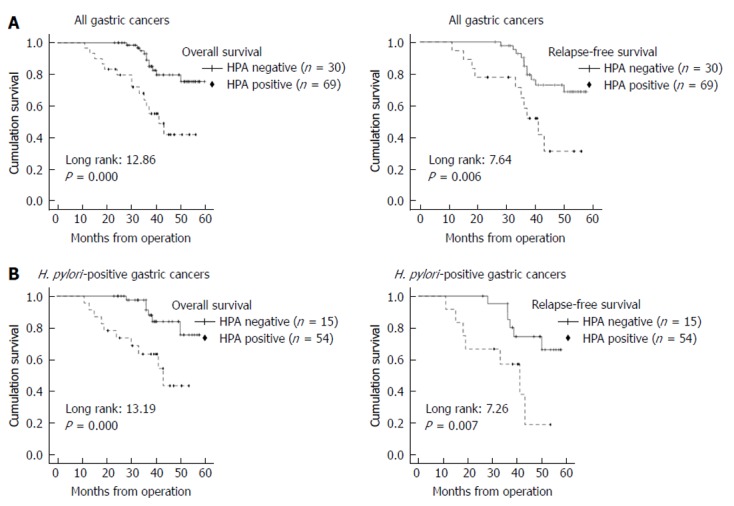
Kaplan-Meier survival plots for overall survival and relapse-free survival according to heparanase expression and Helicobacter pylori infection status. A: Heparanase (HPA) expression status (negative or positive) and the prognosis of all gastric cancer cases. HPA positive staining includes all cases of 1+, 2+, and 3+. HPA positive expression detected by immunohistochemical staining significantly predicts poor overall survival and relatively poor relapse-free survival; B: Kaplan-Meier survival according to HPA status in Helicobacter pylori positive gastric cancer cases. HPA positive expression significantly predicts poor overall survival as well as relapse-free survival. HPA: Heparanase; H. pylori: Helicobacter pylori.
H. pylori infection, MAPK, and prognosis
MAPK positive expression in GC cannot predict overall survival (P = 0.063) or relapse-free survival (P = 0.163). However, in H. pylori-infected GCs, MAPK positive expression was a relatively significant factor for predicting poor prognosis (overall survival, P = 0.007), but did not predict relapse-free survival (P = 0.557) (Figure 4).
Figure 4.
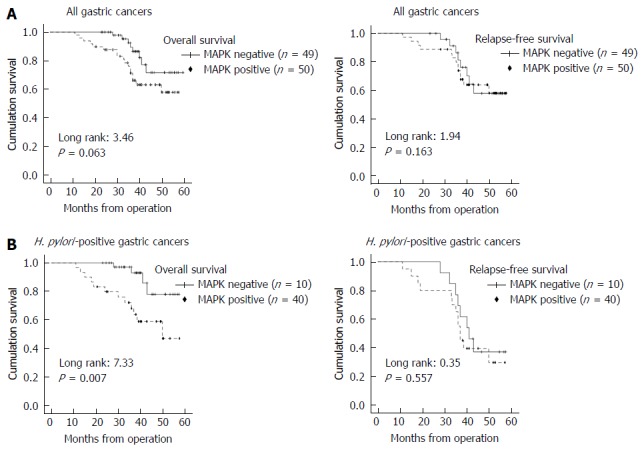
Kaplan-Meier survival plots for overall survival and relapse-free survival according to mitogen-activated protein kinase expression and Helicobacter pylori infection status. A: Mitogen-activated protein kinase (MAPK) expression status (negative or positive) and prognosis of all gastric cancer cases. MAPK positive staining includes all cases of 1+, 2+, and 3+. MAPK positive expression detected by immunohistochemical staining cannot predict overall survival or relapse-free survival; B: Kaplan-Meier survival according to MAPK status in Helicobacter pylori (H. pylori) positive gastric cancer cases. MAPK positive expression significantly predicts poor overall survival in H. pylori positive gastric cancer cases, but does not predict relapse free survival. MAPK: Mitogen-activated protein kinase; H. pylori: Helicobacter pylori.
Prognostic analysis using the COX regression model
Univariate COX regression analysis: By applying the COX regression model to single factor prognostic analysis, a stepwise regression approach was used. The results showed that there was no correlation between gender, age, and tumour diameter. However, lymph node metastasis in GC patients had a significant influence on prognosis (P = 0.003). The degree of histological differentiation had a positive impact on the prognosis of patients with GC (P = 0.003); the tumour invasion depth was also significantly associated with the prognosis of GC patients (P = 0.015). The level of HPA expression also had a very important role in the prognosis of GC patients (P = 0.000), and MAPK expression also had an influence on the prognosis of GC patients (P = 0.031). Whether H. pylori infection was present in patients with GC had no correlation with prognosis (P = 0.849), but H. pylori infection may play an important role in the initiation of GC. Therefore, the univariate COX regression analysis showed that lymph node metastasis, tissue differentiation, depth of invasion, and HPA expression in GC tissues were significantly correlated with the prognosis of patients (Table 7).
Table 7.
Univariate COX regression analysis
| Variable |
Overall survival |
||
| HR | 95%CI | P value | |
| Gender (man vs women) | 0.952 | 0.498-1.821 | 0.882 |
| Age (≥ 60 vs < 60) | 1.113 | 0.581-2.130 | 0.747 |
| Tumour diameter (≥ 40 mm vs < 40 mm) | 1.285 | 0.684-2.413 | 0.436 |
| Lymph metastasis (yes vs no) | 2.667 | 1.381-5.128 | 0.003 |
| Tissue differentiation (high and medium vs low) | 4.156 | 1.622-10.656 | 0.003 |
| Invasive depth (T3/4 vs T1/2) | 2.464 | 1.189-5.104 | 0.015 |
| HPA expression (positive vs negative) | 3.282 | 1.968-7.475 | 0.000 |
| MARK expression (positive vs negative) | 2.083 | 1.068-4.065 | 0.031 |
| H. pylori (positive vs negative) | 1.065 | 0.556-2.045 | 0.849 |
HPA: Heparanase; MAPK: Mitogen-activated protein kinase; H. pylori: Helicobacter pylori; HR: Hazard ratio; CI: Confidence interval.
Multivariate COX regression analysis: The COX regression multivariate model was used for analysis. The results showed that age, sex, tumour diameter, MAPK expression, and H. pylori infection were all removed from the model and that lymph node metastasis, tissue differentiation, depth of invasion, and HPA expression were independent prognostic factors that affected the overall survival rate of GC patients. The relative risk of lymph node metastasis in GC tissues [hazard ratio (HR) = 4.443, P = 0.000] indicated that there was a higher risk of death (increased by 6.52 times) in GC patients with lymph node metastasis compared with those without. The lower the tissue differentiation of patients with GC, the higher the risk of death (HR = 3.452, P = 0.013). The greater the invasion of GC, the higher the risk of death [risk ratio (RR) = 2.542, P = 0.015]. The higher the expression of HPA protein in GC tissues, the shorter the survival time of patients (RR = 2.463, P = 0.015) (Table 8)
Table 8.
Multivariate COX regression analysis
| Variable |
Overall survival |
||
| HR | 95%CI | P value | |
| Lymph metastasis (yes vs no) | 4.443 | 2.185-9.036 | 0.000 |
| Tissue differentiation (high and medium vs low) | 3.452 | 1.299-9.176 | 0.013 |
| Invasive depth (T3/4 vs T1/2) | 2.542 | 1.198-5.392 | 0.015 |
| HPA expression (positive vs negative) | 2.463 | 1.195-5.076 | 0.015 |
HPA: Heparanase; HR: Hazard ratio; CI: Confidence interval.
DISCUSSION
HPA has been documented in many primary human tumours[26-28], including GC[29], and is known to have multiple vital functions in accelerating tumour growth, angiogenesis, and tumour metastasis[30-32]. MAPK, including JNK, ERK, and p38 kinase, plays pivotal roles in proliferation, invasion, and migration of cancer cells[33-36]. There are many studies[37-39] that have shown that H. pylori infection leads to increased p38MAPK in GC cells, and it has also been shown[40] that p38MAPK leads to HPA elevation. This study showed that HPA and MAPK expression was significantly higher in patients with GC than that in para-carcinoma and normal gastric tissues. Our research also confirmed that HPA was highly expressed in GC cells, as has been previously reported in the literature[41,42]. To explain the correlation between HPA and MAPK expression and the clinical pathology of GC cases, we first investigated the clinicopathological characteristics. Positive expression of HPA was associated with lymph node metastasis and depth of invasion, but not with age, gender, tumour diameter, or differentiation degree. In addition, positive MAPK expression was only associated with depth of invasion, which illustrates that HPA is involved in the invasion and metastasis of GC and that MAPK is primarily involved in the proliferation of GC cells. This research also suggests that there is a significant correlation between HPA expression and MAPK expression in GC.
H. pylori, as a class I carcinogen, causes invasion, proliferation, and metastasis of GC cells[43,44], but its specific mechanism of action remains unclear. It has been reported[45,46] that infections can cause a HPA elevation. H. pylori, as a bacterium, is associated with various human gastric diseases, especially gastritis and GC, but there has been no evaluation of whether H. pylori infection causes an increase in HPA in GC, which would contribute to the invasion and metastasis of GC. In the present study, it was revealed that H. pylori infection is significantly associated with HPA expression and that a positive H. pylori infection is connected to lymph node metastasis. To further elucidate the effect of H. pylori infection leading to heparanase elevation in GC, MKN-45 GC cells were infected by H. pylori. We showed that HPA expression was the highest at 24 h post H. pylori infection in these GC cells and the abilities of invasion and metastasis were increased when GC cells were infected by H. pylori.
There are many studies[37-39] showing that H. pylori infection leads to increased p38MAPK in GC cells, and it has been shown that[40] p38MAPK leads to elevation of HPA. Therefore, we hypothesized that H. pylori infection causes an increase in HPA in GC via the MAPK pathway. In this study, it was demonstrated that H. pylori infection was significantly associated with MAPK expression and that there was a significant correlation between HPA expression and MAPK expression in GC. In MKN-45 GC cells infected by H. pylori, H. pylori infection significantly enhanced the expression of MAPK. MAPK expression peaked at 60 min post H. pylori infection in MKN-45 cells. Inhibition of MAPK by SB203580 significantly decreased the expression of HPA and the invasion and metastasis of MKN-45 cells infected by H. pylori. Therefore, we speculate that H. pylori infection in GC activates MAPK signalling, leading to the activation of HPA.
HPA expression is a poor prognostic factor in some cancers[47-49], including GC[50,51]. In the present study, it was revealed that positive expression of HPA was able to predict the malignancy of GC due to its correlation with lymphatic metastasis and invasive depth. Beyond that, positive expression of HPA was a poor prognostic factor for overall survival and relapse-free survival compared with HPA negative cases, which was consistent with previously published reports[52]. Especially in GC patients with an obvious H. pylori infection, HPA positive expression indicated a poorer prognosis both in overall survival and in relapse-free survival, which illustrates that HPA is an important factor for the prediction of prognosis and relapse of GC and that H. pylori infection leads to an increase of HPA expression, which can worsen the prognosis of GC and make recurrence more likely. Compared to HPA, positive MAPK expression only predicted prognosis in overall survival of GC patients, which was consistent with previous reports[53], but MAPK expression could not predict a relapse in H. pylori-infected GC. Thus, MAPK expression cannot be used to determine the prognosis of GC patients with H. pylori infection, but a poor prognosis of GC patients with positive HPA expression is associated with H. pylori positive cases, which suggests that therapy against HPA should be taken into account when GC patients are infected with H. pylori.
Univariate COX regression analysis showed that lymph node metastasis, degree of histological differentiation, and invasive depth in GC patients had a significant influence on prognosis, which was identical to the results of previously published reports[54]. Moreover, in the univariate COX regression analysis, the levels of HPA and MAPK expression also had an influence on the prognosis of GC patients. Similarly, multivariate COX regression analysis showed that lymph node metastasis, tissue differentiation, depth of invasion, and HPA protein expression level were independent prognostic factors that affected the overall survival rate of GC patients. In addition to using the clinical characteristics to judge prognosis, such as lymph node metastasis, tissue differentiation, and depth of invasion, HPA is still an important factor as a biomarker to judge the prognosis of GC, which is consistent with the report by Takaoka et al[51].
In conclusion, the results of the current study demonstrate that H. pylori infection is not only the primary factor involved in GC but is also involved in the invasion and metastasis of GC by upregulating HPA expression, which is likely mediated via activation of the MAPK signalling pathway. HPA is an important factor for predicting the prognosis and relapse of GC, and H. pylori infection increases HPA expression, which makes the prognosis of GC more aggressive and recurrence more likely, suggesting that therapy against HPA should be taken into consideration when GC patients are infected with H. pylori.
ARTICLE HIGHLIGHTS
Research background
The underlying mechanism that Helicobacter pylori (H. pylori) infection results in gastric cancer (GC) is still unknown. Heparanase (HPA) leads to the invasion and metastasis of GC. However, it is not clear whether H. pylori infection in GC increases HPA expression. Such finding suggests that HPA may become a therapeutic target for GC with H. pylori infection.
Research motivation
Although there have been increasing numbers of studies indicating that H. pylori infection results in GC, the underlying mechanism is still unknown. HPA is expressed in many tumours and leads to the invasion and metastasis of tumour, especially in GC. H. pylori infection can induce the development of GC by activating mitogen-activated protein kinase (MAPK) which is closely related to the expression of HPA. However, it is not clear whether MAPK is involved in the regulation of HPA expression following H. pylori infection that leads to GC.
Research objectives
To detect the mechanisms of H. pylori infection in the invasion and metastasis of GC.
Research methods
Immunohistochemistry method was used to detect H. pylori infection and HPA and MAPK expression in GC tissue, and their association with the clinical features of GC was analysed with SPSS 22.0. Kaplan-Meier method and COX proportional models were used to analyse prognosis. HPA and MAPK expression in MKN-45 cells infected with H. pylori was analysed using Western blot.
Research results
This study demonstrates that H. pylori infection increases HPA expression in GC, which is likely mediated via activation of the MAPK signaling pathway.
Research conclusions
The current study shows that H. pylori infection is involved in the invasion and metastasis of GC by upregulating HPA expression, which is likely mediated via activation of the MAPK signaling pathway. HPA is an important factor for predicting the prognosis and relapse of GC with H. pylori infection.
Research perspectives
HPA may become a therapeutic target for GC with H. pylori infection.
ACKNOWLEDGMENTS
We thank Dr. Zhong-Tian Bai for his critical reading of this manuscript. The authors appreciate Wen-Ting He for technical assistance.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was approved by the Ethics Review Board of the First Hospital of Lanzhou University.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Data sharing statement: No additional unpublished data are available.
Peer-review started: August 13, 2018
First decision: August 27, 2018
Article in press: October 5, 2018
P- Reviewer: Cheng H, Xie X S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Bian YN
Contributor Information
Li-Ping Liu, The Second Clinical Medical School of Lanzhou University, Lanzhou 730000, Gansu Province, China; Department of Critical Care Medicine, The First Hospital of Lanzhou University, Lanzhou 730000, Gansu Province, China; Department of Critical Care Medicine, The Donggang District of First Hospital of Lanzhou University, Lanzhou 730030, Gansu Province, China.
Xi-Ping Sheng, Institute of Epidemiology and Health Statistics, School of Public Health, Lanzhou University, Lanzhou 730000, Gansu Province, China.
Tian-Kui Shuai, Department of Critical Care Medicine, The Donggang District of First Hospital of Lanzhou University, Lanzhou 730030, Gansu Province, China.
Yong-Xun Zhao, Department of Surgical Oncology, The First Hospital of Lanzhou University, Lanzhou 730000, Gansu Province, China.
Bin Li, Department of Critical Care Medicine, The First Hospital of Lanzhou University, Lanzhou 730000, Gansu Province, China.
Yu-Min Li, Key Laboratory of Digestive System Tumors of Gansu Province, The Second Clinical Medical School of Lanzhou University, Lanzhou 730000, Gansu Province, China. liym@lzu.edu.cn.
References
- 1.Piazuelo MB, Epplein M, Correa P. Gastric cancer: an infectious disease. Infect Dis Clin North Am. 2010;24:853–869, vii. doi: 10.1016/j.idc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Wang YH, Lv ZF, Zhong Y, Liu DS, Chen SP, Xie Y. The internalization of Helicobacter pylori plays a role in the failure of H. pylori eradication. Helicobacter. 2017;22 doi: 10.1111/hel.12324. [DOI] [PubMed] [Google Scholar]
- 4.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Wang QL, Cheng DD, Xu WT, Lu NH. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front Cell Infect Microbiol. 2016;6:159. doi: 10.3389/fcimb.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Zou D, Ma X, Chen J, Shi X, Gong Y, Man X, Gao L, Zhao Y, Wang R, et al. Epidemiology of peptic ulcer disease: endoscopic results of the systematic investigation of gastrointestinal disease in China. Am J Gastroenterol. 2010;105:2570–2577. doi: 10.1038/ajg.2010.324. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Zhou YZ, Li XY, Tang Z, Zhu HM, Yang Y, Chhetri JK. Seroepidemiology of Helicobacter pylori infection in elderly people in the Beijing region, China. World J Gastroenterol. 2014;20:3635–3639. doi: 10.3748/wjg.v20.i13.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N. Heparanase: From basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016;29:54–75. doi: 10.1016/j.drup.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivara S, Milazzo FM, Giannini G. Heparanase: a rainbow pharmacological target associated to multiple pathologies including rare diseases. Future Med Chem. 2016;8:647–680. doi: 10.4155/fmc-2016-0012. [DOI] [PubMed] [Google Scholar]
- 10.Nadir Y, Brenner B. Heparanase multiple effects in cancer. Thromb Res. 2014;133 Suppl 2:S90–S94. doi: 10.1016/S0049-3848(14)50015-1. [DOI] [PubMed] [Google Scholar]
- 11.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Ni M, Elli S, Naggi A, Guerrini M, Torri G, Petitou M. Investigating Glycol-Split-Heparin-Derived Inhibitors of Heparanase: A Study of Synthetic Trisaccharides. Molecules. 2016;21 doi: 10.3390/molecules21111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlodavsky I, Iozzo RV, Sanderson RD. Heparanase: multiple functions in inflammation, diabetes and atherosclerosis. Matrix Biol. 2013;32:220–222. doi: 10.1016/j.matbio.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JC, Laloo AE, Singh S, Ferro V. 1H NMR spectroscopic studies establish that heparanase is a retaining glycosidase. Biochem Biophys Res Commun. 2014;443:185–188. doi: 10.1016/j.bbrc.2013.11.079. [DOI] [PubMed] [Google Scholar]
- 15.Jin H, Zhou S. The Functions of Heparanase in Human Diseases. Mini Rev Med Chem. 2017;17:541–548. doi: 10.2174/1389557516666161101143643. [DOI] [PubMed] [Google Scholar]
- 16.Yingying X, Yong Z, Zhenning W, Xue Z, Li J, Yang L, Huimian X. Role of heparanase-1 in gastric carcinoma invasion. Asian Pac J Cancer Prev. 2009;10:151–154. [PubMed] [Google Scholar]
- 17.Meirovitz A, Hermano E, Lerner I, Zcharia E, Pisano C, Peretz T, Elkin M. Role of heparanase in radiation-enhanced invasiveness of pancreatic carcinoma. Cancer Res. 2011;71:2772–2780. doi: 10.1158/0008-5472.CAN-10-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafat I, Pode D, Peretz T, Ilan N, Vlodavsky I, Nisman B. Clinical significance of urine heparanase in bladder cancer progression. Neoplasia. 2008;10:125–130. doi: 10.1593/neo.07875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermano E, Lerner I, Elkin M. Heparanase enzyme in chronic inflammatory bowel disease and colon cancer. Cell Mol Life Sci. 2012;69:2501–2513. doi: 10.1007/s00018-012-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding SZ, Smith MF Jr, Goldberg JB. Helicobacter pylori and mitogen-activated protein kinases regulate the cell cycle, proliferation and apoptosis in gastric epithelial cells. J Gastroenterol Hepatol. 2008;23:e67–e78. doi: 10.1111/j.1440-1746.2007.04912.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen YC, Wang Y, Li JY, Xu WR, Zhang YL. H pylori stimulates proliferation of gastric cancer cells through activating mitogen-activated protein kinase cascade. World J Gastroenterol. 2006;12:5972–5977. doi: 10.3748/wjg.v12.i37.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang Y, Ye W, Huang C, Lou B, Zhang J, Yu D, Huang X, Chen B, Zhou M. Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-κb/Stat3/Bcl-2 signaling pathway. Biochem Biophys Res Commun. 2017;487:820–826. doi: 10.1016/j.bbrc.2017.04.133. [DOI] [PubMed] [Google Scholar]
- 24.Chao W, Deng JS, Li PY, Liang YC, Huang GJ. 3,4-Dihydroxybenzalactone Suppresses Human Non-Small Cell Lung Carcinoma Cells Metastasis via Suppression of Epithelial to Mesenchymal Transition, ROS-Mediated PI3K/AKT/MAPK/MMP and NFκB Signaling Pathways. Molecules. 2017;22 doi: 10.3390/molecules22040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Fang H, Chen H, Jiang X, Fang D, Wang Y, Zhu D. An artificial miRNA against HPSE suppresses melanoma invasion properties, correlating with a down-regulation of chemokines and MAPK phosphorylation. PLoS One. 2012;7:e38659. doi: 10.1371/journal.pone.0038659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Song B, Qin WJ, Zhang G, Zhang R, Luan Q, Pan TJ, Yang AG, Wang H. Heparanase promotes bone destruction and invasiveness in prostate cancer. Cancer Lett. 2008;268:252–259. doi: 10.1016/j.canlet.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Varchalama E, Rodolakis A, Strati A, Papageorgiou T, Valavanis C, Vorgias G, Lianidou E, Antsaklis A. Quantitative analysis of heparanase gene expression in normal cervical, cervical intraepithelial neoplastic, and cervical carcinoma tissues. Int J Gynecol Cancer. 2009;19:1614–1619. doi: 10.1111/IGC.0b013e3181ae3f40. [DOI] [PubMed] [Google Scholar]
- 28.Wu BW, Li DF, Ke ZF, Ma D, Li YJ, Gang D, Zheng ZG, Zhang KJ, Zhang YH. Expression characteristics of heparanase in colon carcinoma and its close relationship with cyclooxygenase-2 and angiogenesis. Hepatogastroenterology. 2010;57:1510–1514. [PubMed] [Google Scholar]
- 29.Ma XM, Shen ZH, Liu ZY, Wang F, Hai L, Gao LT, Wang HS. Heparanase promotes human gastric cancer cells migration and invasion by increasing Src and p38 phosphorylation expression. Int J Clin Exp Pathol. 2014;7:5609–5621. [PMC free article] [PubMed] [Google Scholar]
- 30.Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- 31.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 32.Mogler C, Herold-Mende C, Dyckhoff G, Jenetzky E, Beckhove P, Helmke BM. Heparanase expression in head and neck squamous cell carcinomas is associated with reduced proliferation and improved survival. Histopathology. 2011;58:944–952. doi: 10.1111/j.1365-2559.2011.03834.x. [DOI] [PubMed] [Google Scholar]
- 33.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 34.Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo HM. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res. 2003;63:8330–8337. [PubMed] [Google Scholar]
- 35.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 36.Achkar IW, Abdulrahman N, Al-Sulaiti H, Joseph JM, Uddin S, Mraiche F. Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Transl Med. 2018;16:96. doi: 10.1186/s12967-018-1471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Liu N, Shen B, Zhou L, Wang Y, Wang Y, Sun J, Fan Z, Liu RH. Helicobacter pylori enhances cyclooxygenase 2 expression via p38MAPK/ATF-2 signaling pathway in MKN45 cells. Cancer Lett. 2009;278:97–103. doi: 10.1016/j.canlet.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 38.Kim H, Seo JH, Kim KH. The effect of p38 mitogen-activated protein kinase on mucin gene expression and apoptosis in Helicobacter pylori-infected gastric epithelial cells. Ann N Y Acad Sci. 2003;1010:90–94. doi: 10.1196/annals.1299.014. [DOI] [PubMed] [Google Scholar]
- 39.Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004;84:49–62. doi: 10.1038/sj.labinvest.3700010. [DOI] [PubMed] [Google Scholar]
- 40.Che G, Wang Y, Zhou B, Gao L, Wang T, Yuan F, Zhang L. Knockdown of heparanase suppresses invasion of human trophoblasts by activating p38 MAPK signaling pathway. Dis Markers. 2018;2018:1–10. doi: 10.1155/2018/7413027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng L, Jiang G, Mei H, Pu J, Dong J, Hou X, Tong Q. Small RNA interference-mediated gene silencing of heparanase abolishes the invasion, metastasis and angiogenesis of gastric cancer cells. BMC Cancer. 2010;10:33. doi: 10.1186/1471-2407-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang W, Nakamura Y, Tsujimoto M, Sato M, Wang X, Kurozumi K, Nakahara M, Nakao K, Nakamura M, Mori I, et al. Heparanase: a key enzyme in invasion and metastasis of gastric carcinoma. Mod Pathol. 2002;15:593–598. doi: 10.1038/modpathol.3880571. [DOI] [PubMed] [Google Scholar]
- 43.Shichijo S, Hirata Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J Gastroenterol. 2018;24:2163–2172. doi: 10.3748/wjg.v24.i20.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung KS, Leung WK. Risk of gastric cancer development after eradication of Helicobacter pylori. World J Gastrointest Oncol. 2018;10:115–123. doi: 10.4251/wjgo.v10.i5.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matan M, King D, Peled E, Ackerman S, Bar-Lavi Y, Brenner B, Nadir Y. Heparanase level and procoagulant activity are reduced in severe sepsis. Eur J Haematol. 2018;100:182–188. doi: 10.1111/ejh.12997. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg R, Meirovitz A, Hirshoren N, Bulvik R, Binder A, Rubinstein AM, Elkin M. Versatile role of heparanase in inflammation. Matrix Biol. 2013;32:234–240. doi: 10.1016/j.matbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vornicova O, Naroditsky I, Boyango I, Shachar SS, Mashiach T, Ilan N, Vlodavsky I, Bar-Sela G. Prognostic significance of heparanase expression in primary and metastatic breast carcinoma. Oncotarget. 2017;9:6238–6244. doi: 10.18632/oncotarget.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu B, Wang Q, Shi Y, Lu S, Qu H, Wang L, Cui J. Significance of heparanase in metastatic lymph nodes of cervical squamous cell cancer. Oncol Lett. 2017;13:3219–3224. doi: 10.3892/ol.2017.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vornicova O, Boyango I, Feld S, Naroditsky I, Kazarin O, Zohar Y, Tiram Y, Ilan N, Ben-Izhak O, Vlodavsky I, et al. The prognostic significance of heparanase expression in metastatic melanoma. Oncotarget. 2016;7:74678–74685. doi: 10.18632/oncotarget.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Xu S, Tan Q, Liu L. High expression of heparanase-2 is an independent prognostic parameter for favorable survival in gastric cancer patients. Cancer Epidemiol. 2013;37:1010–1013. doi: 10.1016/j.canep.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Takaoka M, Naomoto Y, Ohkawa T, Uetsuka H, Shirakawa Y, Uno F, Fujiwara T, Gunduz M, Nagatsuka H, Nakajima M, et al. Heparanase expression correlates with invasion and poor prognosis in gastric cancers. Lab Invest. 2003;83:613–622. doi: 10.1097/01.lab.0000067482.84946.bd. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Yang J, Han X, Zhao Z, DU L, Yu T, Wang H. Overexpression of heparanase multiple antigenic peptide 2 is associated with poor prognosis in gastric cancer: Potential for therapy. Oncol Lett. 2012;4:178–182. doi: 10.3892/ol.2012.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He X, Liu Z, Xia Y, Xu J, Lv G, Wang L, Ma T, Jiang L, Mou Y, Jiang X, et al. HOXB7 overexpression promotes cell proliferation and correlates with poor prognosis in gastric cancer patients by inducing expression of both AKT and MARKs. Oncotarget. 2017;8:1247–1261. doi: 10.18632/oncotarget.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwon YH. Long-Term Clinical Efficacy and Safety of Endoscopic Submucosal Dissection for Early Gastric Cancer in Korea. Gut Liver. 2018;12:371–372. doi: 10.5009/gnl18216. [DOI] [PMC free article] [PubMed] [Google Scholar]


