Abstract
The significance of perineural invasion (PNI) present in penile cancer (PC) is controversial. In order to clarify the predictive role of PNI in the inguinal lymph node (ILN) metastases (ILNM) and oncologic outcome of patients, we performed this meta-analysis and systematic review. The search of PubMed, Embase, and Web of Science was conducted for appropriate studies, up to 20 January 2018. The pooled odds ratio (OR) and hazard ratio (HR) with their 95% confidence interval (CI) were applied to evaluate the difference in ILNM and oncologic outcome between patients present with PNI and those who were absent. A total of 298 in 1001 patients present with PNI were identified in current meta-analysis and systematic review. Significant difference was observed in ILNM between PNI present and absent from patients with PC (OR = 2.98, 95% CI = 2.00–4.45). Patients present with PNI had a worse cancer-specific survival (CSS) (HR = 3.58, 95% CI = 1.70–7.55) and a higher cancer-specific mortality (CSM) (HR = 2.20, 95% CI = 1.06–3.82) than those cases without PNI. This meta-analysis and systematic review demonstrated the predictive role of PNI in ILNM, CSS, and CSM for PC patients.
Keywords: meta-analysis, penile cancer, perineural invasion, systematic review
Introduction
Penile cancer (PC), as a rare malignancy, was estimated to affect approximately 2000 Americans in 2017 [1]. However, In South America, Southeast Asia, and parts of Africa, the incidence of PC reaches up to 10–20% in male malignancies [2]. Pathologically, squamous cell carcinoma (SCC) is the predominant subtype of PC, and rare clear cell carcinoma along with extramammary Paget disease (EMPD) of penis had also been described [3–6]. Clinically, the lymph node metastasis (LNM) always indicates a poor prognosis. And, 5-year survival rate may diminish to 80% in patients with inguinal lymph nodes (ILNs) metastases (ILNM), 0–33% with pelvic lymph nodes (PLNs) involvement [7,8].
Traditionally, radical ILN dissection (ILND) is standard-of-care for patients with positive ILNs, which can significantly improve the outcome of 80% patients with low-volume positive ILNs [9]. Unfortunately, radical ILND leads to approximately 40–70% of patients suffering major or minor complications. Some studies indicated that 30–50% of patients with clinically positive ILNs were treated based on ‘risk factors’ provided by guidelines, such as clinical ILNs status, tumor grade and stage [10,11]. Meanwhile, occult metastases exist in approximately 25% patients with non-palpable nodes and surgical lymph node staging proposed for intermediate (pT1G2) and high risk patients (any T2 or grade 3–4) according to the 2014 European Association of Urology (EAU) guidelines undoubtedly increases the medical expenses and suffering of PC patients [12,13].
Although some pathologic factors predicting LNM and prognosis of PC, including tumor grade, stage, lymphovascular invasion, LNM, and extranodal extension, were widely accepted and included in the 8th AJCC TNM staging, the outcomes predicted by them are not very accurate [10–12]. Many other risk factors, such as subtypes of PC, the thickness and depth of neoplasm, site, patterns of growth and invasion, perineural invasion (PNI), and positive margins of resection, were also found to hold predictive value in PC, but results from studies were discordant. So, these pathologic factors were limited in clinical practice.
PNI in PC, defined by Velazquez et al. [14] that the perineural space was open and clear, and the tumor invaded outside the perineural room, was first introduced in 2009 EAU Guidelines within many other potential pathologic risks for LNM [15]. However, it did not acquire sufficient attention even in the 2014 EAU Guidelines and 2017 National Comprehensive Cancer Network (NCCN) Guidelines until in the updates in 8th AJCC TNM staging guideline. The PNI was included to divide T1a and T1b, suggesting PNI was a significantly risk factor of LNM and outcome of patients [16]. Nevertheless, PNI still is a controversial predictor in PC patients. Several studies indicated that PC patients with PNI carried a higher risk of ILNM [14,17–19]. On the contrary, several researchers took an opposite opinion [5,20,21]. Meanwhile, limited studies demonstrated PNI as an independent prognostic factor in multivariate analysis. Hence, in order to evaluate its predictive role in prognosis and assess the relationship between PNI and ILNM, a meta-analysis and systematic review was performed by including all eligible studies to distinguish this statistical evidence.
Materials and methods
Search strategy
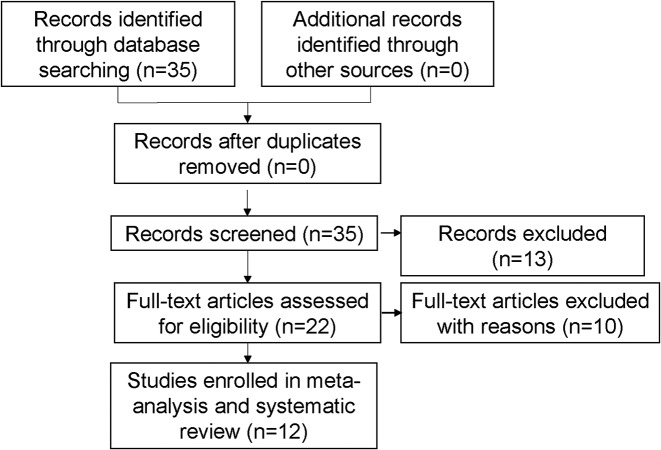
A systematic research was conducted on PubMed, Embase, and Web of Science for potential articles, according to ‘PRISMA’ guideline (till 20 January 2018) [22]. The following medical subjects and combinations were applied: (‘PC’, ‘penile neoplasms’, ‘penile cancer’, ‘penile carcinoma’ or ‘the squamous of penis’), (‘perineural invasion’, ‘perineural infiltration’, or ‘PNI’). Furthermore, we also manually screened the reference list of additional papers. A flow diagram of the research selection process is illustrated in Figure 1.
Figure 1. Study selection process.
Inclusion criteria and exclusion criteria
Studies included had to meet the following criteria: (i) retrospective studies detailing lymph nodes state and outcome of PC patients, (ii) patients were pathologically diagnosed with PC with or without PNI, and (iii) sufficient data could be extracted from included articles. Conversely, the exclusion criteria were as follows: (i) no available or complete data found in included studies, (ii) non-original researches, and (iii) overlapped data appearing in studies.
Data extraction
All worthy data extracted from eligible studies were identified by two independent researchers (X.Z. and F.Q.). The examination of results was undertaken by two investigators (R.Z. and S.W.). The useful data from each study were recorded as follows: age, follow-up period, number of patients, pathologic subtypes, number of PNI patients, number of ILND patients, number of ILNM patients, and hazard ratio (HR)/odds ratio (OR) with 95% confidence intervals (CI) of oncologic outcome. Moreover, the quality of all eligible studies were evaluated by Newcastle–Ottawa Scale.
Statistical analysis
Stata software (version 12.0; Stata Corp LP, College Station, TX) was applied to statistical analysis. The association of PNI with ILNM in patients with PC was assessed by ORs with 95% CIs. For analyzing the role of PNI in outcome of PC patients, the HR and its 95% CI calculated by raw data supplied in article or directly reported by included researches were synthesized, and when HR or its 95% CI was not directly offered, the method described by Altman and Bland [23] was adapted to calculate one of them based on its P-value. If authors offered the results of multivariable and univariable analyses, we recognized the former. Heterogeneity assumption was verified by Galbraith plot, and when heterogeneity appeared, it was more appropriate to adopt the random-effects model other than fixed-effects model to estimate the pooled ORs/HRs. Additionally, a sensitivity analysis was conducted to confirm the robustness of the pooled results, during which data from each individual study was sequentially removed. Moreover, Begg’s funnel plot was conducted to evaluate the publication bias between the studies [24]. Two-tailed P-values were considered statistically significant when less than 0.05.
Results
Studies’ characteristics
A total of 1001 PC patients, 298 patients present with PNI, were identified in current meta-analysis and systematic review for a future evaluation [5,6,14,17–21,25–28]. In addition to SCC patients, there were 41 cases with EMPD eligible for analysis. Those whose age ranged from 52 to 88 years were treated between 1980 and 2016. In these 12 included studies, PC patients came from America [19], U.K. [28], Australia [5], China [18,26], Brazil [14,17,19,21], and Paraguay [14,19]. In addition, the detailed characteristics of enrolled studies were summarized in Table 1 [5,6,14,17–21,25–28]. The association between PNI and ILNM was reported by eight studies [5,14,17–21], but one study was excluded from this meta-analysis because of limited data [6]. Another two studies evaluated the role of PNI in cancer-specific survival (CSS) and cancer-specific mortality (CSM) of patients, listing HR and its 95% CI in multivariable Cox regression, respectively. Moreover, the HR or 95% CI of CSS and CSM was calculated in two articles according to the method described by Altman and Bland [23] based on P-value and HR/95% CI [23,26,27]. Additionally, there were two authors providing the raw data of articles, so we calculated the CSS and overall survival (OS) with HR along with their 95% CI in one study, and OS with HR and 95% CI in another article, using multivariable Cox regression [20,17]. Shu et al. [18] and Sanchez et al. [6] described the number of deaths in patients with and without PNI, which were not applied to meta-analyses for outcome of patients.
Table 1. Detailed characteristics of the studies included in this meta-analysis and systematic review.
| First author | Year | Country | Recruitment period | Study design | Age (mean/median) | Follow-up (mean/ median) | No. pts | Pathology | No. PNI pts | No. ILNM pts | Outcomes | NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNI (P) group | PNI (A) group | PNI (P) group death | PNI (A) group death | |||||||||||
| Ornellas [21] | 2008 | Brazil | 1996–2007 | Retrospective | 57 | 74 | 196 | SCC | 44 | 23 | 47 | NR | 8 | |
| Velazquez [14] | 2008 | Brazil + Paraguay | NR | Retrospective | Mean: 55 | NR | 134 | SCC | 48 | 33 | 33 | NR | 8 | |
| Chaux [19] | 2010 | U.S.A. + Paraguay + Brazil | NR | Retrospective | Mean: 62 | 85 | 45 | SCC | 10 | 6 | 6 | NR | 7 | |
| Gonzaga-Silva [17] | 2012 | Brazil | 1999–2010 | Retrospective | Mean: 65 | 31.9 | 16 | SCC | 11 | 3 | 1 | OS:HR 0.462, 95% CI (0.180–33.618) | 6 | |
| Mannweiler [5] | 2013 | Austria | 1993–2013 | Retrospective | NR | 47 | 76 | SCC | 11 | 6 | 6 | 2 | NR | 7 |
| Mentrikoski [20] | 2014 | U.S.A. | 1990–2012 | Retrospective | 62 | 20 | 59 | SCC | 25 | 12 | 14 | CSS:HR 5.287, 95% CI (0.484–57.752); OS:HR 2.424, 95% CI (0.790–7.439) | 8 | |
| Vassallo [25] | 2015 | Brazil | NR | Retrospective | 52 | NR | 122 | SCC | 48 | NR | CSS:HR 2.47, 95% CI (0.9840–6.2078); DFS:HR 2.78, 95% CI (0.9628–8.0687) | 6 | ||
| Shu [18] | 2015 | China | 2004–2013 | Retrospective | 65 | 36 | 41 | EMPD | 5 | 4 | 10 | 2 | 11 | 6 |
| Geng [26] | 2015 | China | 1997–2009 | Retrospective | 66.5 | 52 | 43 | SCC | 8 | NR | CSS:HR 8.24, 95%CI (1.8306–37.09) | 6 | ||
| Sanchez [6] | 2016 | Paraguay | NR | Retrospective | 88 | 12 | 3 | SCC | 3 | 1 | NR | 2 | 0 | 8 |
| Cunha [27] | 2016 | Brazil | 1980–2014 | Retrospective | 55 | 73 | 149 | SCC | 40 | NR | CSM:HR 1.879, 95% CI (0.945–3.736); ACM:HR 1.553, 95% CI (0.901–2.676) | 7 | ||
| Albersen [28] | 2017 | U.K. | 2005–2016 | Retrospective | 61.9 | 33.7 | 117 | SCC | 45 | NR | LR: HR 2.9713, 95% CI (0.8154–10.8276); CSM:HR 3.1615, 95% CI (0.5604–17.8371) | 7 | ||
Abbreviations: ACM, all cause mortality; DFS, disease-free survival; LR, local recurrence; NOS, Newcastle–Ottawa Scale; NR, not reported; PNI(A), PNI (absent); PNI(P), PNI (present); pts, patients.
ILN metastases
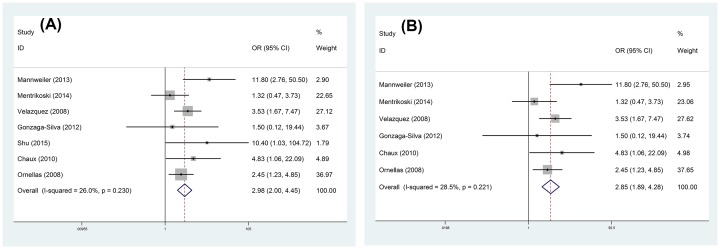
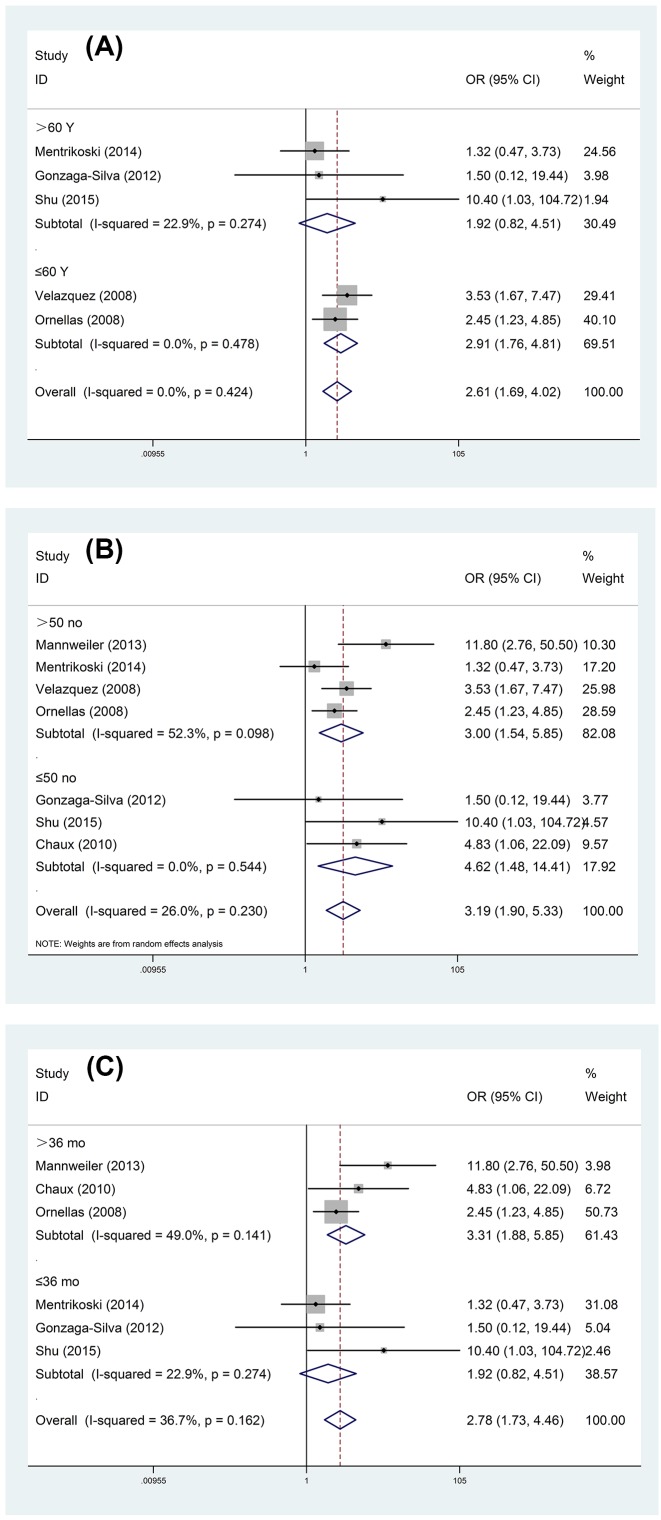
Significant difference was observed in ILNM between PNI present and absent from patients with PC (OR = 2.98, 95% CI = 2.00–4.45) (Figure 2A) after seven studies were synthesized, and patients with PNI undertook a higher risk of ILNM. For PNI in SCC, the main subtype of PC, the risk of ILNM was also apparent (OR = 2.85, 95% CI = 1.89–4.28) (Figure 2B). Meanwhile, we carried out subgroup analysis according to the age of patients, follow-up time, and sample size (Table 2 and Figure 3). Our meta-analysis demonstrated that the predictive value of PNI in ILNM only showed in studies with patients younger than 60 years old (OR = 2.91, 95% CI = 1.76–4.81) and follow-up time longer than 36 months (OR = 3.31, 95% CI = 1.88–5.85).
Figure 2. Forest plots of predictive role of PNI in ILNM for PC: (A) various kinds of PC and (B) SCC of PC.
Table 2. Detailed result of stratified analysis of the role of PNI in ILNM according to the age of patients, sample size of study, and follow-up time of research.
| Analysis | Number of studies (number of patients) | OR (95% CI) | P | Model | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | Phet | |||||
| Subgroup 1 | ||||||
| Age (years) | ||||||
| ≤60 | 2 (330) | 2.91 (1.76–4.81) | 0.000 | Fixed | 0 | 0.478 |
| >60 | 3 (116) | 1.92 (0.82–4.51) | 0.134 | Fixed | 22.9 | 0.274 |
| Subgroup 2 | ||||||
| Sample size (no) | ||||||
| ≤50 | 3 (102) | 4.62 (1.48–14.41) | 0.008 | Random | 0 | 0.544 |
| >50 | 4 (465) | 3.00 (1.54–5.85) | 0.001 | Random | 52.3 | 0.098 |
| Subgroup 3 | ||||||
| Follow-up time (months) | ||||||
| ≤36 | 3 (116) | 1.92 (0.82–4.51) | 0.134 | Fixed | 22.9 | 0.274 |
| >36 | 3 (317) | 3.31 (1.88–5.85) | 0.000 | Fixed | 49 | 0.141 |
Abbreviation: no, number.
Figure 3. Forest plots of stratified analysis of the role of PNI in ILNM: (A) age of patients; (B) sample size of study; (C) follow-up time of reaearch.
Oncologic outcomes of PC patients
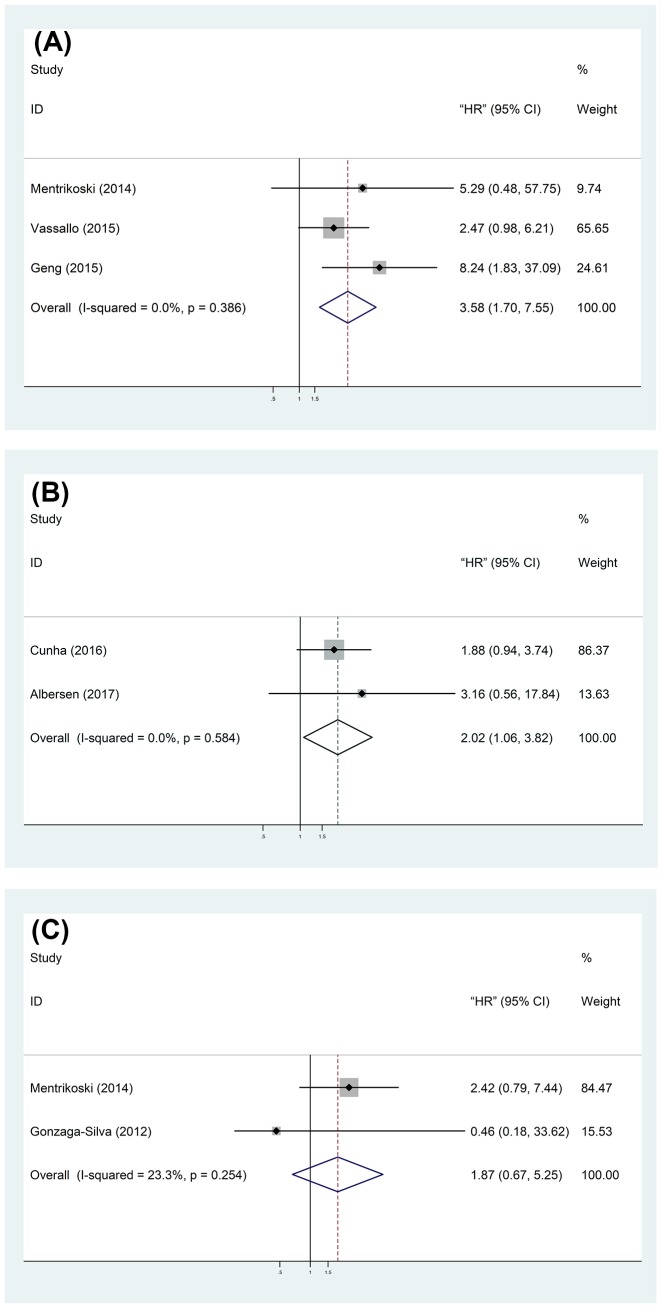
Disease-free survival (DFS), all cause mortality (ACM), or local recurrence (LR) was respectively reported in single study to assess the relationship between PNI and outcome of PC patients, which were not used for outcome analysis. Our result showed that patients present with PNI held a worse CSS (HR = 3.58, 95% CI = 1.70–7.55) (Figure 4A) and a higher CSM (HR = 2.02, 95% CI = 1.06–3.82) (Figure 4B) than those without PNI. However, no significant difference was observed in OS of patients with or without PNI (HR = 1.87, 95% CI = 0.67–5.25) (Figure 4C).
Figure 4. Forest plots of the association between PNI and prognosis: (A) CSS; (B) CSM; (C) OS.
Test of heterogeneity、sensitivity and publication analysis
No obvious heterogeneity was demonstrated. The pooled OR for ILNM (P=0.230 for PC, P=0.221 for SCC), and pooled HR for CSS (P=0.386), CSM (P=0.584), OS (P=0.254) were conducted by utilizing fixed-effort model.
The sensitivity analysis again confirmed the low heterogeneity of the whole studies.
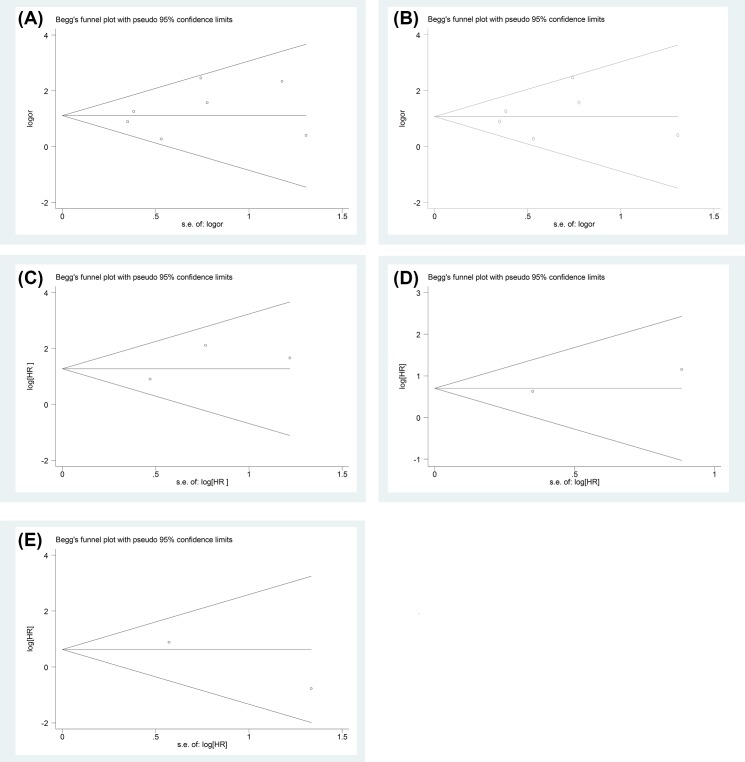
Begg’s funnel plot was also used to evaluate the publication bias of the studies, and the shape had no evidence of obviously asymmetrical, no significant publication bias appearing (Figure 5). Hence, the outcome of this meta-analysis was statistically robust.
Figure 5. Funnel plot for all studies included in this meta-analysis.
Funnel plot assessing predictive role of PNI in ILNM for various kinds of PC ((A) Begg’s test, P=0.548) and SCC ((B) Begg’s test, P=0.707) and association between PNI and CSS ((C) Begg’s test, P=1), CSM ((D) Begg’s test, P=1) or OS ((E) Begg’s test, P=1).
Discussion
Amongst all of the PC patients, approximately 15% of the cases are less than 50 years old [29]. Traditional radical ILND leads to considerable complications. In fact, nearly 50% of patients with palpable nodes and those 75% with clinical negative lymph nodes could be free from the lymph node dissection and concomitant morbidities [10]. For prognosis, LNM and the distant metastases are the most significant predictors [13]. PC patients with pN1 could be able to have 79–89% of 5-year CSS, 7–60% for pN2 and 0–7% for pN3 [30]. Obviously, the difference in CSS is visible even in same risk group. Accordingly, investigating novel pathological predictors of LNM and prognosis is urgent and necessary in adoption of appropriate treatments, and PNI newly incorporated in the newest 8th AJCC TNM staging is promising.
There are small branches of peripheral nerves discovered in Buck’s fascia, preputial dartos, and corpus spongiosum. It is apparent of morphologic discrimination of PNI and not difficult to identify this lesion [31]. Nonetheless, the similarity between PNI and nerve trapped in tumor deserves more attention. The predictive role of PNI for ILNM in SCC invading 5–10 mm was first well demonstrated in a multicenter retrospective study by Velazquez et al. [14] with multivariate logistics regression models in 2008. On contrast, Ornellas et al. [21] from Brazil failed to find that PNI was an independent predictor for ILNM in the same year. The reason for these two contradicted consequence may lie in small number of cases, various therapeutic methods, and heterogeneous pathologic methodologies, which highlight the necessity of meta-analysis in PNI.
The result of this meta-analysis demonstrated that patients with PNI take a considerable risk of carrying ILNM. A subgroup analysis classified by sample size was performed, regardless of the number of each studies, we found statistical significance between PNI and ILNM. These data could be partly explained by that PNI exists in a considerable portion of PC individuals, 30% in our analysis, and can be identified in tumor mass without difficulty. Moreover, association between PNI and ILNM with significance was merely observed in studies with mean/median follow-up time no less than 36 months, which may be attributed to increased ILNM rate appearing during longer follow-up. Additionally, an interesting and crucial finding with regard to the impact of age in the predictor of PNI for ILNM revealed by stratified analysis, our analysis suggested that when PC patients who were younger than 60 years were present with PNI, the hazard of developing ILMN was apparent and statistically significant compared with individuals older than 60 years. This consequence was consistent with result depicted by Paiva et al. [32] that younger patients took more aggressive behavior of PC, which should receive much attention.
Because of the small number of previous studies, there is no consensus on the PNI and relationship with CSS. This meta-analysis could be able to overcome the shortcomings of single study, limited sample size, and insufficient statistical power, it showed that PC patients with PNI undertook adverse CSS and CSM, but not OS.
The early detection of metastases in lymph nodes is promising. Dynamic sentinel node biopsies (DSNB) has been recommended for clinical node negative patients with intermediate and high-risk stage in Europe [33], and sensitivity reached up to 88 and 90% with use of patent blue in a meta-analysis [13]. Nevertheless, the high expenses precludes its implementation in developing countries. Besides, fine needle aspiration cytology (FNAC) with ultrasound (US) can detect tumor in palpable nodes excellently, rapidly, and easily [9,13]. However, when results of pathology are negative, the option of following procedure is in an embarrassing state. Moreover, the sensitivity of conventional radiological examination, such as US, computed tomography (CT) and MRI, for detecting metastases in ILNs is disappointing [34–36]. Recently, positron emission tomography (PET)/CT, a promising radiological examination, demonstrated excellent specificity and sensitivity for distinguishing metastases in palpable nodes of 100 and 90% repactively, whose utility is restricted by limited reliable evidence [37]. Additionally, lymphotrophic nanoparticle-enhanced MRI (LNMRI) showed the promise of implementation, which needs further follow-up [38]. Hence, PNI plays an important role in predicting metastases in lymph nodes and outcome of PC patients in this situation.
Admittedly, this meta-analysis also had some limitations. First, only 12 relevant researches were included, therefore, higher quality and large sample size clinical studies are needed to strengthen our conclusion, especially in the association between prognosis and PNI. Second, some data of eligible studies published between 1980 and 2016, existing different diagnostic criteria and standards of treatment, which increased bias of this meta-analysis. Third, we estimated HR or 95% CI with method described by Altman and Bland [23], which were not same as reported by authors.
Conclusion
The results of this meta-analysis demonstrated that PNI is a worthy predictor of ILNM and imply a poorer CSS and higher CSM in PC patients. Moreover, attention ought to be given to those patients present with PNI who are younger than 60 years. And, higher quality and large sample size clinical studies are needed to strengthen our conclusion
Abbreviations
- CI
confidence interval
- CSM
cancer-specific mortality
- CSS
cancer-specific survival
- CT
computed tomography
- EAU
European Association of Urology
- EMPD
extramammary Paget disease
- HR
hazard ratio
- ILN
inguinal lymph node
- ILND
ILN dissection
- ILNM
ILN metastasis
- OR
odds ratio
- OS
overall survival
- PC
penile cancer
- PNI
perineural invasion
- US
ultrasound
Author contribution
X.Z. took part in the data extraction, statistical analysis, and drafting of the manuscript. F.Q. helped to recheck the results and revised the manuscript. R.Z. and S.W. assisted in statistical analysis and drafting the manuscript. J.Y. and N.S. designed the study program and took responsibility for the integrity of the data and the accuracy of the data analysis. Yamin W., Yichun W., C.C., and Yi W. participated in data extraction. All authors read and approved the final manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Research Project of Jiangsu Provincial Commission of Health and Family Planning Commission [grant number Z201601].
References
- 1.Siegel R.L., Miller K.D. and Jemal A. (2017) Cancer statistics, 2017. CA Cancer J. Clin. 66, 7–30 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Backes D.M., Kurman R.J., Pimenta J.M. and Smith J.S. (2009) Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 20, 449–457 10.1007/s10552-008-9276-9 [DOI] [PubMed] [Google Scholar]
- 3.Liegl B. and Regauer S. (2014) Penile clear-cell carcinoma: a report of 5 cases of a distinct entity. Am. J. Surg. Pathol. 28, 1513–1517 10.1097/01.pas.0000141405.64462.2a [DOI] [PubMed] [Google Scholar]
- 4.Epstein J.I., Chubilla A.L., Humpfrey P.A. (2011) AFIP Atlas of Tumor Pathology, American Registry of Pathology, Washington, DC [Google Scholar]
- 5.Mannweiler S., Sygulla S., Tsybrovskyy O., Razmara Y., Pummer K. and Regauer S. (2013) Clear-cell differentiation and lymphatic invasion, but not the revised TNM classification, predict lymph node metastases in pT1 penile cancer: a clinicopathologic study of 76 patients from a low incidence area. Urol. Oncol. 31, 1378–1385 10.1016/j.urolonc.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 6.Sanchez D.F. et al. (2016) Clear cell carcinoma of the penis: an HPV-related variant of squamous cell carcinoma: a report of 3 cases. Am. J. Surg. Pathol. 40, 917–922 10.1097/PAS.0000000000000607 [DOI] [PubMed] [Google Scholar]
- 7.Srinivas V., Morse M.J., Herr H.W., Sogani P.C. and Whitmore W.F. Jr (1987) penile cancer: relation of extent of nodal metastasis to survival. J. Urol. 137, 880–882 10.1016/S0022-5347(17)44281-9 [DOI] [PubMed] [Google Scholar]
- 8.Pandey D., Mahajan V. and Kannan R.R (2006) Prognostic factors in node-positive carcinoma of the penis. J. Surg. Oncol. 93, 133–138 10.1002/jso.20414 [DOI] [PubMed] [Google Scholar]
- 9.Leone A., Diorio G.J., Pettaway C., Master V. and Spiess P.E. (2017) Contemporary management of patients with penile cancer and lymph node metastasis. Nat. Rev. Urol. 14, 335–347 10.1038/nrurol.2017.47 [DOI] [PubMed] [Google Scholar]
- 10.Protzel C., Alcaraz A., Horenblas S., Pizzocaro G., Zlotta A. and Hakenberg O.W. (2009) Lymphadenectomy in the surgical management of penile cancer. Eur. Urol. 55, 1075–1088 10.1016/j.eururo.2009.02.021 [DOI] [PubMed] [Google Scholar]
- 11.Lont A.P., Kroon B.K., Gallee M.P., van Tinteren H., Moonen L.M. and Horenblas S. (2007) Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J. Urol. 177, 947–952 10.1016/j.juro.2006.10.060 [DOI] [PubMed] [Google Scholar]
- 12.Djajadiningrat R.S. et al. (2013) Value of dynamic sentinel node biopsy, ultrasound and fine needle aspiration in the detection of metastatic lymph nodes in penile cancer. Eur. Urol. Suppl. 12, e394–e395 10.1016/S1569-9056(13)60879-9 [DOI] [Google Scholar]
- 13.Hakenberg O.W., Compérat E.M., Minhas S., Necchi A., Protzel C. and Watkin N. (2015) EAU guidelines on penile cancer: 2014 update. Eur. Urol. 67, 142–150 10.1016/j.eururo.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 14.Velazquez E.F. et al. (2008) Histologic grade and perineural invasion are more important than tumor thickness as predictor of nodal metastasis in penile squamous cell carcinoma invading 5 to 10 mm. Am. J. Surg. Pathol. 32, 974–979 10.1097/PAS.0b013e3181641365 [DOI] [PubMed] [Google Scholar]
- 15.Pizzocaro G. et al. (2010) EAU penile cancer guidelines 2009. Eur. Urol. 57, 1002–1012 10.1016/j.eururo.2010.01.039 [DOI] [PubMed] [Google Scholar]
- 16.Paner G.P., Stadler W.M., Hansel D.E., Montironi R., Lin D.W. and Amin M.B. (2018) Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur. Urol, 73, 560–569 10.1016/j.eururo.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 17.Gonzaga-Silva L.F. et al. (2012) Locally advanced penile carcinoma: classic emasculation or testis-sparing surgery? Int. Braz J. Urol. 38, 750–759 10.1590/1677-553820133806750 [DOI] [PubMed] [Google Scholar]
- 18.Shu B., Shen X.X., Chen P., Fang X., Guo Y.L. and Kong Y.Y. (2016) Primary invasive extramammary Paget disease on penoscrotum: a clinicopathological analysis of 41 cases. Hum Pathol. 47, 70–77 10.1016/j.humpath.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Chaux A. et al. (2010) Warty-basaloid carcinoma: clinicopathological features of a distinctive penile neoplasm. Report of 45 cases. Mod. Pathol. 23, 896–904 10.1038/modpathol.2010.69 [DOI] [PubMed] [Google Scholar]
- 20.Mentrikoski M.J., Stelow E.B., Culp S., Frierson H.F. Jr and Cathro H.P. (2014) Histologic and immunohistochemical assessment of penile carcinomas in a North American population. Am. J. Surg. Pathol. 38, 1340–1348 10.1097/PAS.0000000000000124 [DOI] [PubMed] [Google Scholar]
- 21.Ornellas A.A., Nóbrega B.L. and Wei Kin Chin E. (2018) Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J. Urol. 180, 1354–1359 10.1016/j.juro.2008.06.028 [DOI] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A. and Tetzlaff J. (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 23.Altman D.G. and Bland J.M. (2011) How to obtain the confidence interval from a P value. BMJ 343, d2090 10.1136/bmj.d2090 [DOI] [PubMed] [Google Scholar]
- 24.Egger M., Davey S.G. and Schneider M. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassallo J., Rodrigues A.F. and Campos A.H. (2015) Pathologic and imunohistochemical characterization of tumoral inflammatory cell infiltrate in invasive penile squamous cell carcinomas: Fox-P3 expression is an independent predictor of recurrence. Tumour Biol. 36, 2509–2516 10.1007/s13277-014-2864-2 [DOI] [PubMed] [Google Scholar]
- 26.Geng J.H., Huang S.P. and Huang C.Y. (2015) Prognostic factors in Taiwanese patients with penile-invasive squamous cell carcinoma. Kaohsiung J. Med. Sci. 31, 523–528 10.1016/j.kjms.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 27.da Cunha I.W., Souza M.J. and da Costa W.H. (2016) Epithelial-mesenchymal transition (EMT) phenotype at invasion front of squamous cell carcinoma of the penis influences oncological outcomes. Urol. Oncol. 34, 433.e19–433.e26 10.1016/j.urolonc.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 28.Albersen M., Parnham A. and Joniau S. (2017) Predictive factors for local recurrence after glansectomy and neoglans reconstruction for penile squamous cell carcinoma. Urol. Oncol. 17, 30387–30383 [DOI] [PubMed] [Google Scholar]
- 29.Minhas S., Kayes O. and Hegarty P. (2005) What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 96, 1040–1043 10.1111/j.1464-410X.2005.05769.x [DOI] [PubMed] [Google Scholar]
- 30.Novara G., Galfano A. and De Marco V. (2007) Prognostic factors in squamous cell carcinoma of the penis. Nat. Clin. Pract. Urol. 4, 140–146 10.1038/ncpuro0751 [DOI] [PubMed] [Google Scholar]
- 31.Aynaud O., Asselain B. and Bergeron C. (2000) Intraepithelial carcinoma and invasive carcinoma of vulva, vagina and penis in Ile-de-France. Ann. Dermatol. Venereol. 127, 479–483 [PubMed] [Google Scholar]
- 32.Paiva G.R., de Oliveira Araújo I.B. and Athanazio D.A. (2015) Penile cancer: impact of age at diagnosis on morphology and prognosis. Int. Urol. Nephrol. 47, 295–299 10.1007/s11255-014-0875-y [DOI] [PubMed] [Google Scholar]
- 33.Clark P.E., Spiess P.E., Agarwal N.. et al. (2013) penile cancer: clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 11, 594–615 10.6004/jnccn.2013.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stella I., Wayne L. and Tom S. (2010) A prospective study to evaluate the performance of ultrasound with or without fine needle aspiration of inguinal nodes in patients with squamous cell carcinoma of the penis. Asco Genitourinary Cancers Symposium 183, e224 [Google Scholar]
- 35.Horenblas S., van Tinteren H. and Delemarre J.F. (1991) Squamous cell carcinoma of the penis: accuracy of tumor, nodes and metastasis classification system, and role of lymphangiography, computerized tomography scan and fine needle aspiration cytology. J. Urol. 146, 1279–1283 10.1016/S0022-5347(17)38068-0 [DOI] [PubMed] [Google Scholar]
- 36.Kayes O., Allen C. and Li C. (2006) Comparison of diagnostic radiological modalities in predicting inguinal lymph node involvement in penile cancer. Eur. Urol. Suppl. 5, 83 10.1016/S1569-9056(06)60250-9 [DOI] [Google Scholar]
- 37.Schlenker B., Scher B. and Tiling R. (2012) Detection of inguinal lymph node involvement inpenile squamous cell carcinoma by 18F-fluorodeoxyglucose PET/CT: A prospective single-center study. Urol. Oncol. 30, 55–59 10.1016/j.urolonc.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 38.Tabatabaei S., Harisinghani M. and McDougal W.S. (2005) Regional lymph node staging using lymphotropic nanoparticle enhanced magnetic resonance imaging with ferumoxtran-10 in patients with penile cancer. J. Urol. 174, 923–928 10.1097/01.ju.0000170234.14519.19 [DOI] [PubMed] [Google Scholar]