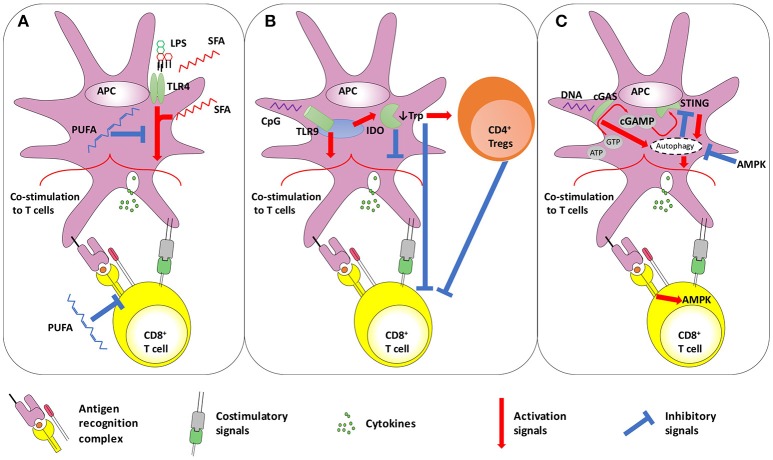
Figure 1.
Schematic representation of the interplay between (A) TLR4 and fatty acid metabolism, (B) TLR9 and IDO, and (C) STING and autophagy. (A) TLR4 activation on APCs improves CD8+ T-cell priming. In addition to LPS, SFA are also thought to trigger TLR4. However, it has also been proposed that SFA act on TLR4-downstream pathways. In contrast, PUFA display anti-inflammatory effects, by dampening both TLR4- and TCR-induced signaling. (B) Dual role of TLR9 stimulation on T-cell activation. The TLR9 ligand CpG shows adjuvant effects, improving the co-stimulation delivered by APCs to T-cells. However, some reports highlighted that the same pathway may also trigger negative regulators of immunity, such as IDO that down-modulates APC-provided co-stimulation and favors Treg activity. Furthermore, IDO mediates tryptophan deprivation, with has negative consequences on T-cell functionality. (C) The autophagy-STING loop. The cytosolic DNA sensors cGAS converts ATP and GTP into the dinucleotide cGAMP, which triggers STING. Both cGAS and STING may promote authophagy, that can be involved in two distinct processes: inducing APC-delivered co-stimulation to T-cells, and STING degradation to avoid its permanent activation. The latter process seems under the control of AMPK, a kinase also acting in downstream TCR signaling in T-cells. AMPK, AMP-activated protein kinase; APC, antigen presenting cell; ATP, Adenosine Triphosphate; cGAMP, cyclic guanosine monophosphate–adenosine monophosphate; cGAS, cGAMP synthase; CpG, CpG oligodeoxynucleotides; GTP, Guanosine Triphosphate; IDO, Indoleamine 2;3-dioxygenase; Trp, tryptophan; LPS, lipopolysaccharide; PUFA, poly-unsaturated fatty acids; SFA, saturated fatty acids; STING, stimulator of interferon genes; TLR, toll like receptor.