Abstract
The efficacy and safety of immune‐checkpoint inhibitors in non‐small cell lung cancer patients with idiopathic pulmonary fibrosis (IPF) remain unknown. Herein, we describe the case of a 62‐year‐old man with multiple pleural tumors and carcinomatous pleurisy. High‐resolution computed tomography indicated usual interstitial pneumonia, and a respiratory function test revealed a restrictive disorder and decreased diffusion capacity. He was diagnosed with lung adenocarcinoma and IPF. After failure of initial chemotherapy, he was treated with nivolumab and achieved a complete response without any sign of exacerbation of IPF. The response to nivolumab has persisted for > 1 year. This is the first report of a non‐small cell lung cancer patient with IPF who has been treated with immune‐checkpoint inhibitors for such a long period and achieved a sustained response.
Keywords: Idiopathic pulmonary fibrosis (IPF), immune‐checkpoint inhibitor, lung adenocarcinoma, nivolumab, PD‐1
Introduction
Although PD‐1 checkpoint inhibitors, including nivolumab, are now standard treatment for previously treated advanced non‐small cell lung cancer (NSCLC),1, 2, 3 patients with coexisting interstitial lung disease (ILD) were excluded from the original clinical trials for fear of exacerbating this condition.4 Idiopathic pulmonary fibrosis (IPF) is a specific form of ILD characterized by chronic progressive fibrosing interstitial pneumonia of unknown cause and is potentially fatal. It has been therefore unclear whether immune‐checkpoint inhibitors (ICIs) are tolerable and efficient treatment options for NSCLC patients with IPF. Herein, we describe the case of a patient with NSCLC and concomitant IPF who has experienced a durable response to nivolumab without exacerbating the IPF.
Case report
A 62‐year‐old man with a smoking history of 20 cigarettes per day for 42 years presented with a cough and fever. High‐resolution computed tomography (HRCT) revealed multiple pleural disseminations and pleural effusion on the left side (Fig 1a). CT‐guided biopsy of a pleural mass confirmed a diagnosis of lung adenocarcinoma, negative for EGFR mutation or EML4‐ALK fusion. Cytological analysis of the pleural effusion revealed malignant adenocarcinoma cells. No distant metastasis was detected by magnetic resonance imaging or positron emission tomography–CT. CT also revealed a subpleural basal‐predominant reticular shadow and traction bronchiectasis with a honeycomb pattern (Fig 1b), indicative of usual interstitial pneumonia (UIP). A respiratory function test revealed a low predicted vital capacity (%VC) of 74% and a reduced diffusing capacity of the lung for CO of 51.1%, indicative of restrictive disorder. The patient had no physical or laboratory findings suggestive of collagen vascular disease or other diseases, and had no history of dust exposure. He was finally diagnosed with lung adenocarcinoma with pleural dissemination and IPF.
Figure 1.
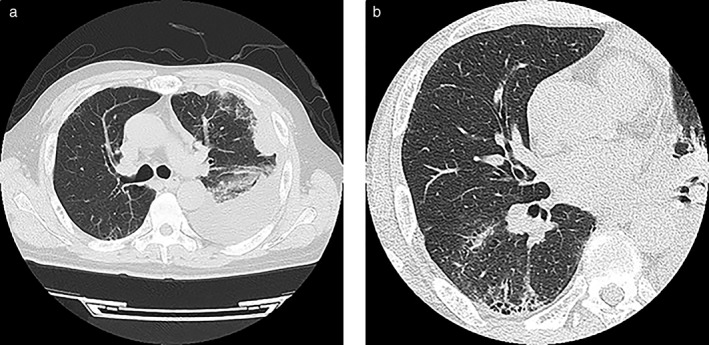
Initial computed tomography images showing (a) multiple pleural disseminations and pleural effusion on the left side, as well as (b) a subpleural basal‐predominant reticular shadow and traction bronchiectasis with a honeycomb pattern.
After drainage of the pleural effusion, the patient underwent combination chemotherapy with cisplatin, pemetrexed, and bevacizumab every four weeks, followed by maintenance chemotherapy with pemetrexed and bevacizumab. HRCT performed after three cycles of maintenance chemotherapy showed disease progression. Immunohistochemistry at diagnosis of the proband detected PD‐L1 expression on 75% of the tumor cells. After receiving fully informed consent regarding the risk of IPF exacerbation, we administered nivolumab as standard second‐line therapy at a dose of 3 mg/kg every two weeks. HRCT revealed a partial response after four cycles, followed by a complete response after 14 cycles (Fig 2a–c). The patient experienced no symptoms related to the existing IPF, and HRCT showed no signs of exacerbation (Fig 2d–f). He has now received nivolumab treatment for > 1 year and achieved a sustained response.
Figure 2.
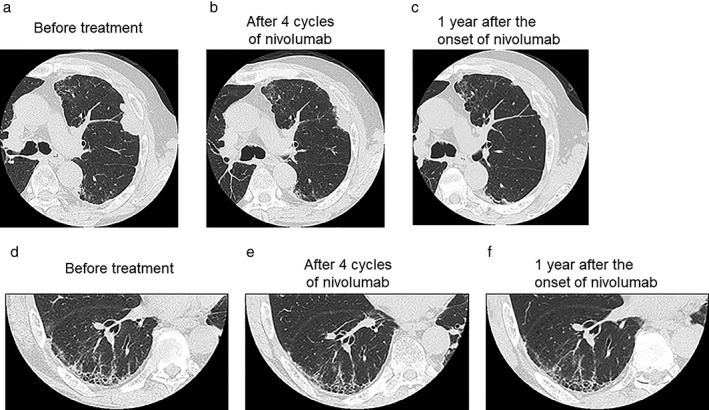
High‐resolution computed tomography images of the (a–c) tumors and (d–f) idiopathic pulmonary fibrosis, (a,d) before and (b,e) after four cycles and (c,f) one year of nivolumab treatment.
Discussion
As far as we are aware, only two previous reports have described ICI treatment in NSCLC patients with IPF. One presented a case of lung squamous cell carcinoma and IPF treated with nivolumab.5 The patient was treated with nivolumab safely for eight months, which resulted in stable disease. The second study prospectively investigated the safety of nivolumab for advanced NSCLC patients with “mild” (defined as a %VC of ≥ 80%) idiopathic interstitial pneumonia with a possible UIP or a pattern inconsistent with UIP on a chest HRCT scan, to exclude the possibility of acute exacerbation.6 None of the six patients studied developed pneumonitis within 12 weeks, three achieved a partial response, and the disease control rate was 100%.
A pulmonary function test revealed that our patient had an apparent restrictive disorder (%VC of < 80%) with reduced diffusion capacity, which we attributed to chronic progression of IPF. However, he has now been receiving nivolumab for > 1 year with a durable complete response and no notable toxicity. IPF is significantly associated with patient characteristics, such as microsatellite instability and smoking history.7, 8, 9 Such characteristics are also associated with a high tumor mutation burden (TMB), a likely independent biomarker for ICI treatment in NSCLC.10 Although no information regarding TMB in NSCLC with IPF is currently available, a high PD‐L1 expression level and substantial TMB might account for the durable response of the proband to nivolumab.
This is the first report of an NSCLC patient with IPF who has experienced a durable response to nivolumab for > 1 year without exacerbation of IPF. The results in this case support the emerging evidence that nivolumab is a feasible option for the treatment of NSCLC, even if complicated by IPF. Prospective studies to investigate the safety and efficacy of ICIs including nivolumab in NSCLC patients with IPF are warranted.
Disclosure
No authors report any conflict of interest.
Contributor Information
Maako Ide, Email: ffyourwish@gmail.com.
Isamu Okamoto, Email: okamotoi@kokyu.med.kyushu-u.ac.jp.
References
- 1. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raghu G, Collard HR, Egan JJ et al An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence‐based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khunger M, Velcheti V. A case of a patient with idiopathic pulmonary fibrosis with lung squamous cell carcinoma treated with nivolumab. J Thorac Oncol 2017; 12: e96–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujimoto D, Morimoto T, Ito J et al A pilot trial of nivolumab treatment for advanced non‐small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer 2017; 111: 1–5. [DOI] [PubMed] [Google Scholar]
- 7. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demopoulos K, Arvanitis DA, Vassilakis DA, Siafakas NM, Spandidos DA. MYCL1, FHIT, SPARC, p16(INK4) and TP53 genes associated to lung cancer in idiopathic pulmonary fibrosis. J Cell Mol Med 2002; 6: 215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiao D, Pan H, Li F, Wu K, Zhang X, He J. Analysis of ultra‐deep targeted sequencing reveals mutation burden is associated with gender and clinical outcome in lung adenocarcinoma. Oncotarget 2016; 7: 22857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carbone DP, Reck M, Paz‐Ares L et al First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med 2017; 376: 2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


