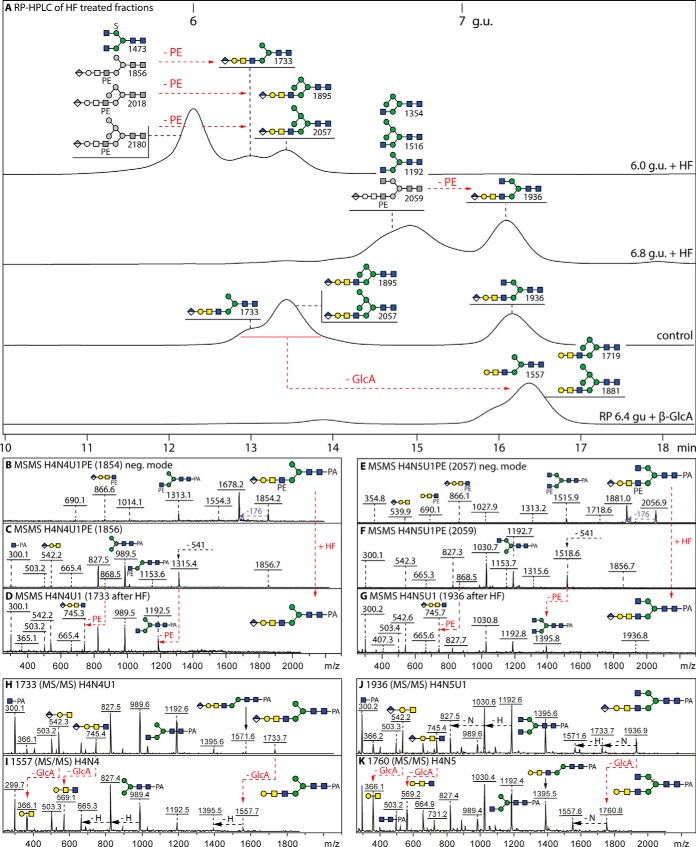
Fig. 6.
RP-HPLC and MS/MS analysis of glycans modified with both glucuronic acid and phosphoethanolamine. A, RP-amide chromatograms of the two hydrofluoric acid (HF) treated 6.0 and 6.8 g.u. fractions are shown compared with a control co-injection of aliquots of 6.4 and 7.2 g.u. glucuronylated glycans. The effect of E. coli β-glucuronidase on the retention time of the 6.4 g.u. control glycans, corresponding to the HF products of the 6.0 g.u. fraction, is also indicated; see supplemental Figs. 10 and 11 for further MS and HPLC data on glucuronidase and HF digests. B–G, The MALDI-TOF MS/MS of untreated glycans in negative (B and E) and positive modes (C and F) and of the HF-treated products (positive mode only; D and G) highlight changes in the fragmentation patterns because of removal of phosphoethanolamine residues (loss of m/z 868 fragments correlating to the later retention times; indicated with red arrows). (H–K) Positive mode MALDI-TOF MS/MS of the 6.4 and 7.2 g.u. glucuronylated glycans before and after β-glucuronidase digestion indicating loss of the m/z 542 and 745 fragments and appearance of ones at m/z 366 and 569. For space reasons, the phosphoethanolamine group is depicted underneath the antenna, although a 6-linkage is assumed based on analogy to insect glycolipids (36–38); the greyscale structures indicate the original elution times, those in color the products'. Based on these analyses, glycans carrying both phosphoethanolamine and glucuronic acid are estimated to represent 30% of these particular anionic fractions. It is to be noted that sulfated glycans (e.g. m/z 1473) are resistant to hydrofluoric acid treatment, unlike those modified with phosphomono- or diesters.