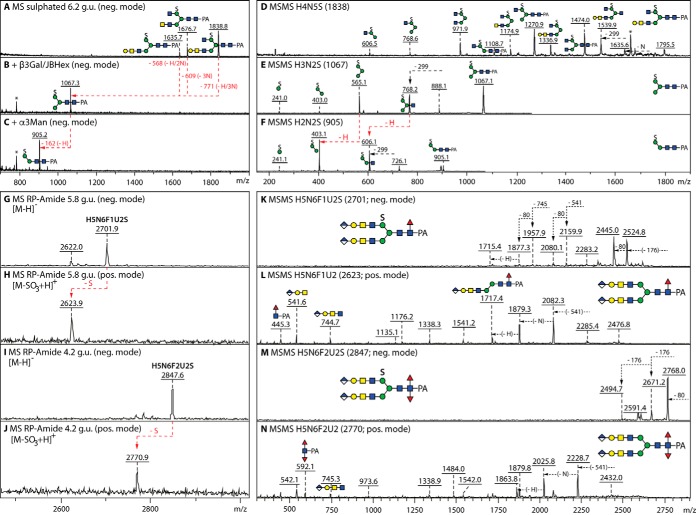
Fig. 7.
Glycosidase digestion and MALDI-TOF MS/MS analysis of sulfated N-glycans. A–C, Negative mode MALDI-TOF MS of the 6.2 g.u. anionic fraction before and after combined β1,3-galactosidase (β3Gal) and jack bean hexosaminidase (JBHex) digestion and subsequent α1,3-mannosidase (α3Man) treatment; the removal of galactose and HexNAc residues results in a single m/z 1067 product from which the α1,3-mannose can be digested, thereby localizing the sulfate to the α1,6-mannose as in other monosulphated glycans from insects. D–F, Negative mode MALDI-TOF MS/MS of a sulfated glycan and of its digestion products. G–N, MS and MS/MS in negative and positive modes of sulfated glycans modified with also fucose and glucuronic acid; in contrast to the ability of phosphorylated glycans to ionize in both positive and negative modes (54), sulfated structures are detectable in the negative mode as [M-H]− and the indicated in-source fragmentation in positive ion mode results in the presence of an ion of 78 mass units lower than the negative ion detected in the same fraction. MS/MS of the [M-SO3+H]+ ion aided definition of the core modifications (m/z 446 and 592 mono- and difucosylated Y-fragments), whereas the sulfation of the 6-linked mannose is assumed by analogy to the smaller structures and to the permethylation data in Supplemental Fig. 3.