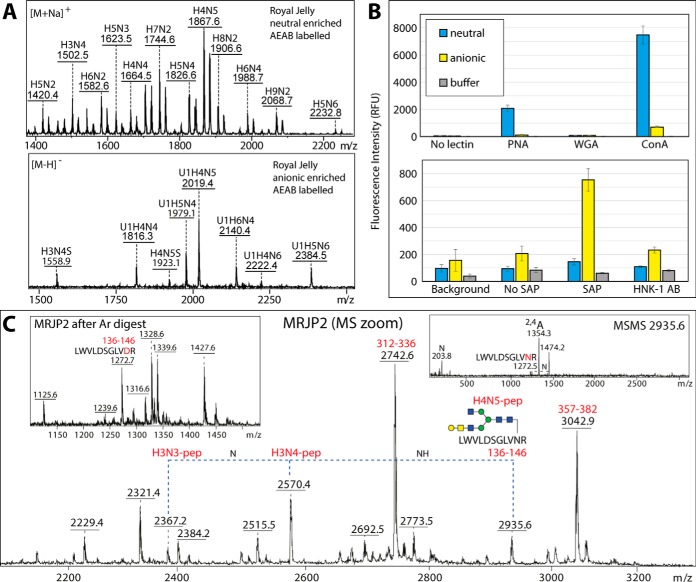
Fig. 9.
A, MALDI-TOF MS of AEAB-labeled royal jelly neutral and anionic N-glycans; refer to supplemental Fig. S14 for further selected MS and MS/MS data. B, The binding of peanut aggulutin (PNA) to neutral and anionic N-glycans and of the human pentraxin serum amyloid P (SAP) and the anti-L2/HNK-1 antibody clone 412 (HNK-1) to anionic N-glycans is proven by probing the AEAB-labeled N-glycan pools on NHS-modified glass slides (see corresponding MALDI-TOF MS); the chart indicates the uncorrected fluorescence values with the standard deviations (green or red channels; mean of 10 spots) as compared with concanavalin A (as a nonspecific lectin) and to negative controls (spotting buffer or no primary lectin, antibody or pentraxin). The recognition by PNA, SAP and anti-L2/HNK-1 correlates, respectively, with the presence of “pseudo” T antigen epitopes in both pools and of phosphoethanolamine and terminal glucuronic acid in the anionic pool as proven by the analyses of the PA-labeled glycans, but the binding of the glycans to wheat germ agglutinin (WGA) is minimal. As shown in supplemental Fig. S14, binding to PNA decreases and to WGA increases after β-galactosidase treatment, whereas binding to anti-L2/HNK-1 is β-glucuronidase sensitive. Refer to supplemental Fig. S13A for Western blotting data with PNA, WGA and SAP. C, A zoomed-in region of the MRJP2 spectrum (see supplemental Fig. S13D) containing ions corresponding to a peptide modified with different complex N-glycans, including one with a T antigen epitope of the type recognized by PNA; the insets show respectively a region of the spectrum after PNGase Ar-treatment and one example MS/MS showing the 2,4A cross-ring GlcNAc-containing fragment for the m/z 2935 glycopeptide. Raw mzXML files corresponding to the full MRJP2 peptide spectra before and after PNGase Ar treatment as well as two MS/MS glycopeptide spectra are included in the supplementary zip archive.