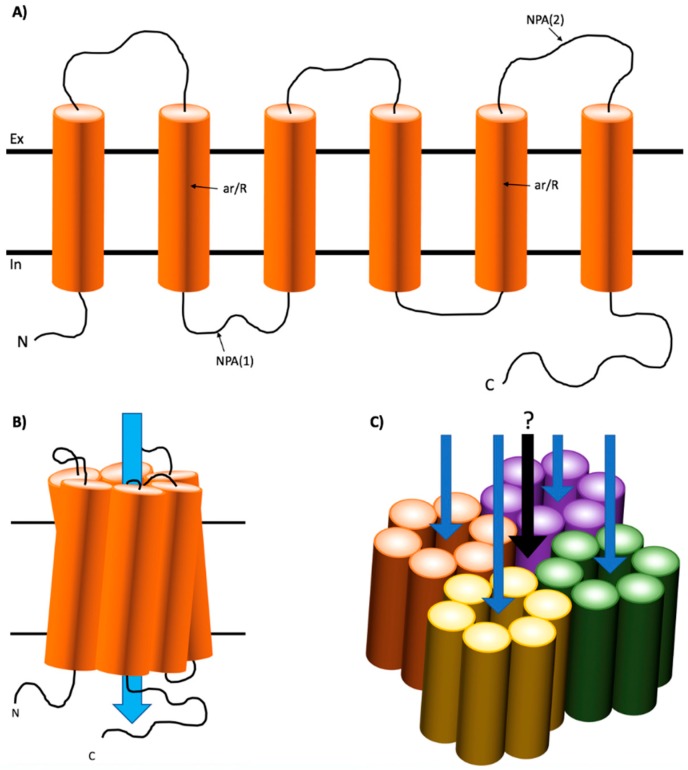
Figure 1.
The secondary, tertiary, and quaternary structures of Aquaporin proteins. (A) The secondary structure of aquaporins contains six a-helices connected by three extracellular and two intracellular loops. Loops B and E contain NPA motifs and the fifth a-helices contain ar/R motifs, which is described in the text. (B) All six α-helices exist in a closely associated tertiary monomer structure with both ar/R motifs interacting at opposite sides of the pore and both NPA motifs interacting within the membrane. The route of water passage exists in the transmembrane pore formed through the center of the three-dimensional barrel. (C) AQP monomers homotetermerize and create a five-pore quaternary structure. However, the function of the central pore, formed by the space between all four monomers, remains largely unknown.