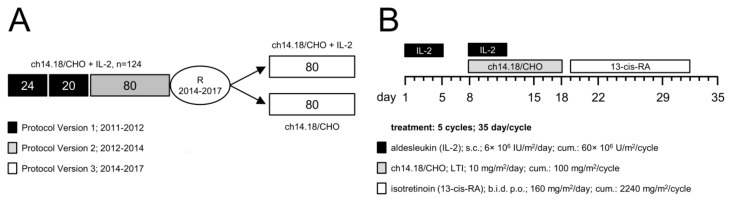
Figure 1.
Schematic overview of the treatment schedule and the time line of the LTI study. (A) The LTI study (EudraCT-Number: 2009-018077-31) was planned as a single-arm study and amended in 2014 to address a randomized question. From 2011 to 2014, 124 pts were recruited in the single-arm phase, and 2 × 80 pts were recruited from 2014 to 2017 in the randomized phase. The single-arm phase consisted of a dose-finding (protocol version 1; 24 + 20 pts) and a dose confirmation cohort (protocol version 2; 80 pts), leading to a total of 124 pts in that part or the trial; (B) 122 of 124 enrolled NB pts received up to five treatment cycles (35 d/cycle) according to the following treatment protocol (2 pts progressed prior to first antibody application): IL-2 (aldesleukin; black horizontal bar) was given once a day for five days (s.c., d1–5, 6 × 106 IU/m2/d), followed by a combined application of IL-2 once a day (s.c., d8–12, 6 × 106 IU/m2/d) with a 10 days continuous infusion of ch14.18/CHO (i.v., d8–18, 10 mg/m2/d; grey horizontal bar). Starting on d19, treatment was continued with 13-cis-retinoic acid (isotretinoin; white horizontal bar) given twice a day (b.i.d) for the next 14 days (p.o., d19–32). Cumulative doses of IL-2, ch14.18/CHO and 13-cis-RA per cycle were 60 × 106 IU/m2, 100 mg/m2 and 2240 mg/m2, respectively.