Fig. 2.
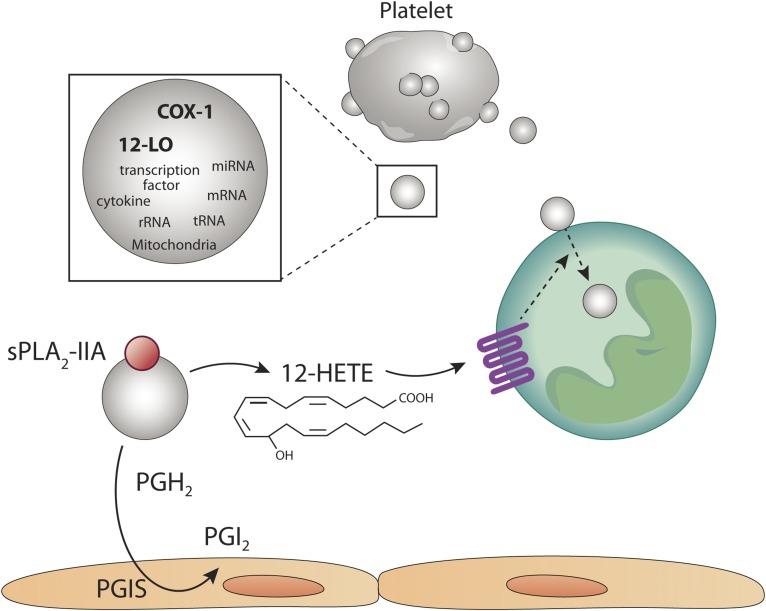
Platelet-derived EVs participate in intercellular communication through their eicosanoid content. Platelets are highly proficient at releasing EVs, such as microvesicles (microparticles). EVs from activated platelets contain a broad cargo, which includes cytokines, transcription factors, cytokines, mitochondria, and nucleic acid. Of importance is the content in active cyclooxygenase-1 (COX-1) and 12-lipoxygenase (12-LO). In the presence of secreted phospholipase A2 (sPLA2) in an inflammatory milieu, platelet-derived EVs generate arachidonic acid, which can be metabolized into prostaglandin H2 (PGH2) and subsequently into prostacyclin (PGI2) by the PGI synthase (PGIS) in endothelial cells. AA liberated by platelet-derived EVs can also undero lipoxygenation by the 12-LO itself present in platelet-derived EVs. Moreover, exogenous AA can be metabolized by platelet-derived EVs by 12-LO. 12-hydroxyeicosatetranoic acid (12-HETE) is generated and promotes the internalization of platelet EVs by neutrophils, thereby permitting efficient transfer of cargo. The internalization of platelet-EVs is therefore tightly regulated by the eicosanoid 12-HETE.