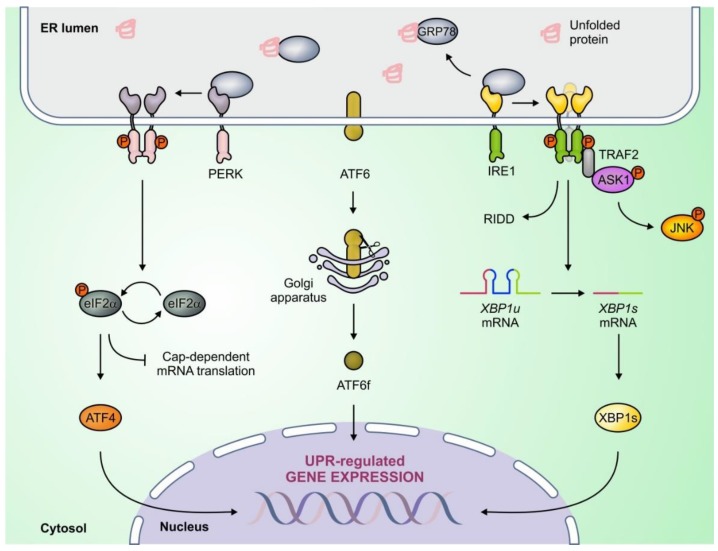
Figure 2.
The unfolded protein response (UPR) is mediated by PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1) signaling. Unfolded proteins within the endoplasmic reticulum (ER) lumen lead to activation of ER stress sensors by sequestering glucose-regulated protein 78 kDa (GRP78). ATF4, ATF6f, and spliced x-box binding protein 1 (XBP1s) are adaptive transcription factors activated by the PERK, ATF6, and IRE1 signaling branches respectively, and promote expression of chaperones and protein degradation pathway components. The UPR also engages degradation of cytosolic RNA (regulated IRE1 dependent decay (RIDD) function) and activation of c-Jun N-terminal kinase (JNK) through IRE1, and inhibition of global protein synthesis through PERK.