Abstract
One hypothesized explanation for the recent slowing of declines in heart disease death rates is the generational shift in the timing and accumulation of risk factors. However, directly testing this hypothesis requires historical age-group-specific risk factor data that do not exist. Using national death records, we compared spatiotemporal patterns of heart disease death rates by age group, time period, and birth cohort to provide insight into possible drivers of trends. To do this, we calculated county-level percent change for five time periods (1973–1980, 1980–1990, 1990–2000, 2000–2010, 2010–2015) for four age groups (35–44, 45–54, 55–64, 65–74), resulting in eight birth cohorts for each decade from the 1900s through the 1970s. From 1973 through 1990, few counties experienced increased heart disease death rates. In 1990–2000, 49.0% of counties for ages 35–44 were increasing, while all other age groups continued to decrease. In 2000–2010, heart disease death rates for ages 45–54 increased in 30.4% of counties. In 2010–2015, all four age groups showed widespread increasing county-level heart disease death rates. Likewise, birth cohorts from the 1900s through the 1930s experienced consistently decreasing heart disease death rates in almost all counties. Similarly, with the exception of 2010–2015, most counties experienced decreases for the 1940s birth cohort. For birth cohorts in the 1950s, 1960s, and 1970s, increases were common and geographically widespread for all age groups and calendar years. This analysis revealed variation in trends across age groups and across counties. However, trends in heart disease death rates tended to be generally decreasing and increasing for early and late birth cohorts, respectively. These findings are consistent with the hypothesis that recent increases in heart disease mortality stem from the beginnings of the obesity and diabetes epidemics. However, the common geographic patterns within the earliest and latest time periods support the importance of place-based macro-level factors.
Keywords: Heart disease mortality, County, Trends, Spatiotemporal, Birth cohort, Age group
1. Introduction
Epidemiologic evidence is clear that heart disease incidence and severity are driven by life course risk factors including smoking, hypertension, and obesity, especially when those risk factors occur in childhood and early adulthood (Lloyd-Jones et al., 2006; Reilly and Kelly, 2011). As the duration of exposure to these risk factors lengthens and occurs earlier in life, individuals are put at higher risk of incident heart disease and, ultimately, of death resulting from heart disease.
Higher individual-level risks of heart disease mortality resulting from life course exposures are then reflected in population-level surveillance data as higher heart disease death rates. Following over forty years of declines, heart disease death rates have leveled or increased in recent years (Sidney et al., 2016; Wilmot et al., 2015). One hypothesized explanation for the recent change in trends is a shift in the life-stage timing and accumulated experience of risk factors that differ across generations and birth cohorts (Ford and Capewell, 2007; Lloyd-Jones, 2016; Olshansky et al., 2005; Sidney et al., 2016). For example, the emergence of the ‘obesity epidemic’ in the 1980’s represented the beginning of a population shift in a key risk factor such that obesity was prevalent younger in life and experienced for longer times than preceding generations (Flegal et al., 2012; Ford et al., 2003; Hales et al., 2017; Imes and Burke, 2014; Mitchell et al., 2011). Similar national increases have occurred for metabolic syndrome, hypertension, and diabetes (Egan et al., 2010; Ford et al., 2004; Harris et al., 1998; Mainous et al., 2007). However, the health consequences of the shifting prevalence of these risk factors across generations are delayed, taking years to manifest as increased heart disease mortality (Flegal et al., 2012; Ford et al., 2003; Hales et al., 2017; Imes and Burke, 2014; Twig et al., 2016). Consequently, directly testing the hypothesis that recent unfavorable changes in heart disease mortality result from differences in lifetime exposure to risk factors (such as obesity and diabetes) requires historical age-group-specific risk factor prevalence data. These surveillance data are simply not available for the populations or geography necessary to respond.
In the absence of historical risk factor data, age-group-specific surveillance data may provide indirect insight into the potential roles of risk factors as drivers of trends in heart disease death rates. With surveillance data (which are typically reported by age group), we can define birth cohorts (and corresponding cohort-related life course exposures) as combinations of age groups in sequential calendar years. Although birth years are reported in vital statistics and are appropriate for use in age-period-cohort analysis of cross-sectional rates (Kramer et al., 2014), their use in calculating change in rates leads to algebraically overlapping age groups within birth cohorts and overlapping birth cohorts with age groups. In turn, this overlap means that age group and birth cohort effects in trends cannot be separated. However, by holding one group fixed (e.g. age group), age group and birth cohort-specific results may be interpreted simultaneously, rather than individually. While individuals within each birth cohort are certainly not identical, birth cohorts may share average life course risk factor exposures, and thus serve as proxies for population-level life course exposure data.
Through this approach, we may then examine the consistency of mortality trends across time periods, age groups, and birth cohorts, allowing this statistical method to indirectly support or refute hypothesized population-level drivers of changing death rates. First, the similarity of trends in age group-specific death rate within a given time period may indicate macro-level factors that similarly influence all age groups (Rodgers et al., 2018). Although more distal than traditional risk factors, macro-level factors have been found to strongly influence heart disease death rates and should not be ignored (Cunningham et al., 2017; Harper et al., 2011; Marmot, 2018; Patel et al., 2016; Rodgers et al., 2018; Zajacova and Montez, 2017). Secondly, similarity of patterns of death rate trends within birth cohorts and across time periods and age groups may indicate cohort-specific exposures, such as traditional heart disease risk factors. These traditional risk factors (e.g. obesity, diabetes) have been found to be strongly associated with birth cohorts (Reither et al., 2009) and, therefore, have been hypothesized to be the cause of recent increases in heart disease mortality (Ford and Capewell, 2007; Lloyd-Jones, 2016; Olshansky et al., 2005; Sidney et al., 2016).
Examining only national trends may mask geographic and population variation in both heart disease death rates and trends over time (Casper et al., 2016), including many counties that have experienced increasing heart disease death rates since 2010 (Vaughan et al., 2017). Thus, incorporating county-level data permits examination of geographic variation in the age and cohort specific patterns of heart disease death rates. These geographic patterns may then provide additional support to the roles of socioeconomic and environmental factors that could influence trends in heart disease mortality.
Therefore, this analysis leveraged county-level age-group-specific heart disease mortality data to examine spatiotemporal patterns of trends in heart disease death rates by calendar time, age group, and birth cohorts. Through this method, we then interpret these results to suggest potential drivers of recent increases in county-level heart disease mortality, including both proximate biologic and behavioral risk factors as well as macro-level social determinants of health.
2. Methods
2.1. Heart disease mortality data
The study population for this analysis included US residents, ages 35 through 74, stratified by four age groups (35–44, 45–54, 55–64, 65–74). We obtained annual counts of heart disease deaths per county per age group from 1973 through 2015 from the National Vital Statistics System (NVSS) of the National Center for Health Statistics (NCHS). Deaths from heart disease were defined as those for which the underlying cause of death was “diseases of the heart” according to the 8th, 9th, and 10th revisions of the International Classification of Diseases (ICD) (ICD-8: 390–398, 402, 404, 410–429; ICD-9: 390–398, 402, 404–429; ICD-10: I00-I09, I11, I13, I20-I51). Comparability ratios between ICD versions are close to one, indicating consistency of this definition over the study period (Anderson et al., 2001; Klebba and Scott, 1980). As data for 1972 were based on a 50% sample of deaths, use of this continuous time period (1973–2015) ensures that all data examined are based on a census of deaths in the United States.
The unit of analysis was the county. Given changes in county definitions during the study period (e.g., the creation of new counties), a single set of 3098 counties from the contiguous lower 48 states based on the most recent county definitions was used for the entire study period. We used NCHS estimates for annual county-level populations (National Center for Health Statistics, 2016).
2.2. Estimated heart disease death rates
We estimated age group-specific, county-level heart disease death rates using a previously described Bayesian multivariate space-time conditional autoregressive model (Quick et al., 2018, 2017). By borrowing strength both spatially and temporally, as well as between age groups, these models generate precise, reliable rates even in the presence of small case counts and small populations (Quick et al., 2017; Vaughan et al., 2015). We fit this model with a Markov chain Monte Carlo (MCMC) algorithm using user-developed code in the R programming language. This code, and an example of its use, may be found here: https://sites.google.com/site/harryq/code. We estimated national and county-level heart disease death rates for each age group as the medians of the posterior distributions defined by the MCMC iterations.
More specifically, we modeled Yikt, the number of deaths due to heart disease in county i and age group k during year t from a population of size nikt, using a Poisson distribution of the form Yikt ~ Pois(nikt γikt), where λikt denotes the heart disease mortality rate. To model λikt, we assume , where βkt is a random intercept for each group for each year with a vague N(0,100) prior, Zikt is a spatiotemporal random effect that incorporates correlation between groups, and is a variance parameter with a weakly informative gamma prior (Waller et al., 1997).
To account for these various sources of dependence, we model Zikt using the multivariate space-time conditional autoregressive (MSTCAR) model of Quick et al. (2018). A special case of the multivariate CAR (MCAR) model of (Gelfand and Vounatsou, 2003), our MSTCAR model shrinks the random effects for each county toward the values in neighboring counties. Similarly, temporal structure is accounted for within the MSTCAR model by shrinking estimates toward adjacent years using an approach similar to a standard autoregressive order 1 (AR(1)) model with a beta prior. Finally, correlations between groups are estimated via an unstructured covariance matrix with an inverse Wishart prior (Waller et al., 1997).
We ran the MCMC algorithm with four chains for 6000 iterations, diagnosing convergence via trace plots for many of the model parameters and discarding the first 3000 iterations as burn-in. We generated estimates based on posterior medians, and 95% credible intervals were obtained by taking the 2.5- and 97.5-percentiles from the thinned post-burn-in samples.
2.3. Calculating annual percent change
We estimated relative annual percent change in county-level heart disease death rates for five time periods (1973–1980, 1980–1990, 1990–2000, 2000–2010, 2010–2015) using Poisson regression including all years within each interval. Using relative change, rather than absolute change, allowed results to be compared across age groups and time periods. These comparisons would not be possible when using absolute change because of large variation in heart disease death rates across age groups and over time. This method permitted all rates to inform estimates of percent change, which is not true when calculating percent change using differences in rates between the beginning and end of each time period. Using the specified intervals allowed us to account for potential non-linearity in trends and to make consistent comparisons across counties. We restricted the last interval to recent years (2010–2015), because during this time period national rates began to plateau and many county-level rates were increasing (Sidney et al., 2016; Vaughan et al., 2017; Wilmot et al., 2015).
Following estimation of rates described in the previous section, separate regression models were run for each county using rates for all years within each time period for each MCMC iteration. Specifically, for MCMC iteration m, county i, and age group k at year t, the Poisson model was ln(nikt λiktm) = β0ikm + βiktm year + ln(nikt), where nikt λiktm represents the estimated number of heart disease deaths. The annual percent change was then calculated as 100(eβ1iklm –1). We then used the median across all MCMC distributions as the estimated percent change for each combination of county, age group, and time period.
2.4. Birth cohorts
The birth cohorts were defined using combinations of the five time periods and four age groups noted above (Fig. 1). Given 10-year age groups and 10-year time periods, some combinations of age groups and time periods have identical distributions of possible birth years (e.g. ages 65–74 dying in 2000–2010, ages 55–64 dying in 1990–2000, and ages 45–54 dying in 1980–1990 were all born between the years of 1926 and 1945, with 80% of possible birth years being in the 1930s), leading to 14 unique groups of birth years (Fig. 1). However, as some of these 14 groups have largely overlapping birth years, we reduced these groups of birth years into eight birth cohorts based on birth decades from the 1900s through the 1970s. This simplification can be seen in Fig. 1 with slightly different groups of birth years (shown in the boxes) being included in a single birth cohort (represented on the diagonals).
Fig. 1.
Years of birth (boxes) calculated for all combinations of age groups (columns) and years of death (rows). Text in boxes are the range representing 80% of possible birth years and the median birth year for the given combination of age and year of death. Birth cohorts are on the diagonals.
3. Results
3.1. National trends in heart disease death rates
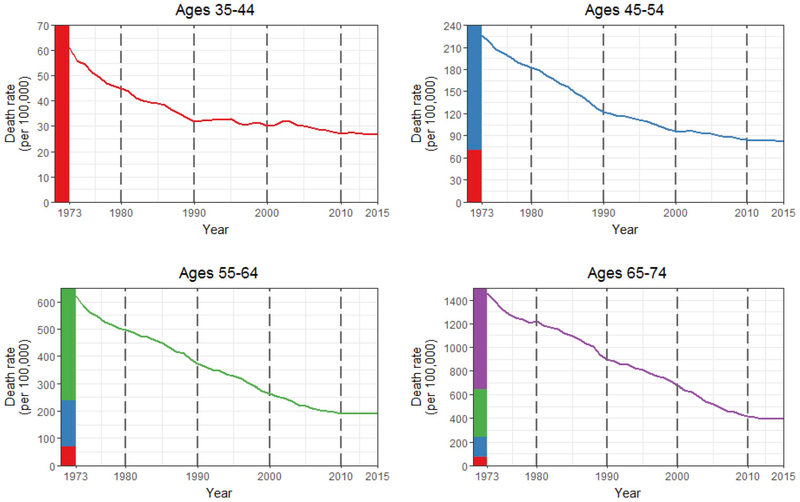
Overall, national heart disease death rates declined for all four age groups (Fig. 2). The flattening of national declines, which was observed for all age groups, began earlier in calendar time for younger age groups. Compared to earlier intervals, decreases in heart disease death rates in the most recent interval (2010–2015) were markedly slower or had reversed for all age groups (annual percent change of −0.2% (95% CI: −0.5, 0.2), −0.3% (−0.6, −0.1), 0.4% (0.1, 0.7), −0.8% (−1.3, −0.2) for ages 35–44, 45–54, 55–64, 65–74, respectively) (Table 1).
Fig. 2.
Heart disease mortality by age group, 1973–2015, United States. The vertical dotted lines indicate the intervals used in this paper. Colored bars on the y-axis indicate the different scales of the four graphs.
Table 1.
Summary of annual percent change in county-level heart disease death rates by age group and year of death, United States, 1973–2015. For each section of the table, patterns within age groups across time period are represented by columns, patterns within time period across age groups are represented by rows, and patterns by birth cohort across both age groups and time periods are on the diagonals.
| Statistic/Year of death | Ages 35–44 | Ages 45–54 | Ages 55–64 | Ages 65–74 |
|---|---|---|---|---|
| National annual percent change (95% credible interval) | ||||
| 1973–1980 | −4.3 (−4.9, −3.7) | −3.0 (−3.4, −2.7) | −3.1 (−3.6, −2.6) | −2.6 (−3.3, −1.9) |
| 1980–1990 | −3.2 (−3.5, −2.9) | −3.9 (−4.2, −3.6) | −2.7 (−3.0, −2.4) | −2.8 (−3.2, −2.4) |
| 1990–2000 | −0.7 (−1.0, −0.3) | −2.4 (−2.6, −2.2) | −3.3 (−3.6, −3.0) | −2.7 (−2.9, −2.4) |
| 2000–2010 | −1.3 (−1.9, −0.7) | −1.4 (−1.6, −1.1) | −3.3 (−3.5, −3.0) | −5.0 (−5.2, −4.8) |
| 2010–2015 | −0.2 (−0.5, 0.2) | −0.3 (−0.6, −0.1) | 0.4 (0.1, 0.7) | −0.8 (−1.3, −0.2) |
| Median of county-level annual percent change (Interquartile range) | ||||
| 1973–1980 | −4.0 (−5.2, −2.8) | −3.1 (−3.9, −2.3 | −3.3 (−4.1, −2.5) | −2.6 (−3.4, −2.0) |
| 1980–1990 | −3.1 (−4.1, −2.0) | −3.6 (−4.7, −2.4) | −2.5 (−3.3, −1.6) | −2.6 (−3.3, −1.8) |
| 1990–2000 | 0.0 (−1.1, 1.1) | −2.1 (−3.0, −1.2) | −3.0 (−3.9, −2.2) | −2.6 (−3.3, −1.9) |
| 2000–2010 | −0.9 (−2.2, 0.4) | −0.9 (−2.0, 0.3) | −3.1 (−3.9, −2.1) | −4.6 (−5.5, −3.7) |
| 2010–2015 | 0.1 (−1.5, 1.8) | 0.4 (−0.8, 1.6) | 0.9 (−0.3, 2.2) | −0.3 (−1.4, 0.8) |
| Number of counties with increasing mortality (Percent) | ||||
| 1973–1980 | 24 (0.8) | 15 (0.5) | 17 (0.5) | 18 (0.6) |
| 1980–1990 | 161 (5.2) | 92 (3.0) | 168 (5.4) | 87 (2.8) |
| 1990–2000 | 1519 (49.0) | 158 (5.1) | 39 (1.3) | 37 (1.2) |
| 2000–2010 | 979 (31.6) | 941 (30.4) | 46 (1.5) | 2 (0.1) |
| 2010–2015 | 1620 (52.3) | 1812 (58.5) | 2141 (69.1) | 1301 (42.0) |
3.2. Geographic patterns in county-level heart disease death rates
At the county level, spatial patterns of heart disease death rates varied across age groups in 1973, but were similar in 2015 (Fig. 3). In 1973, the highest death rates were concentrated in the Southern Atlantic Coast and Appalachia amongst the three youngest age groups, but this geographic pattern spanned the Atlantic Coast, including the Northeast, for ages 65–74. Over time, the concentration of the highest rates for age groups 35–44 and 45–54 moved westward from the Southern Atlantic Coast to the Deep South. For age groups 55–64 and 65–74, the highest rates moved from the Atlantic Coast to the Deep South.
Fig. 3.
County-level heart disease death rates by age group, United States, 1973 and 2015. Categories are quartiles for each map.
3.3. Trends in county-level heart disease death rates within age groups
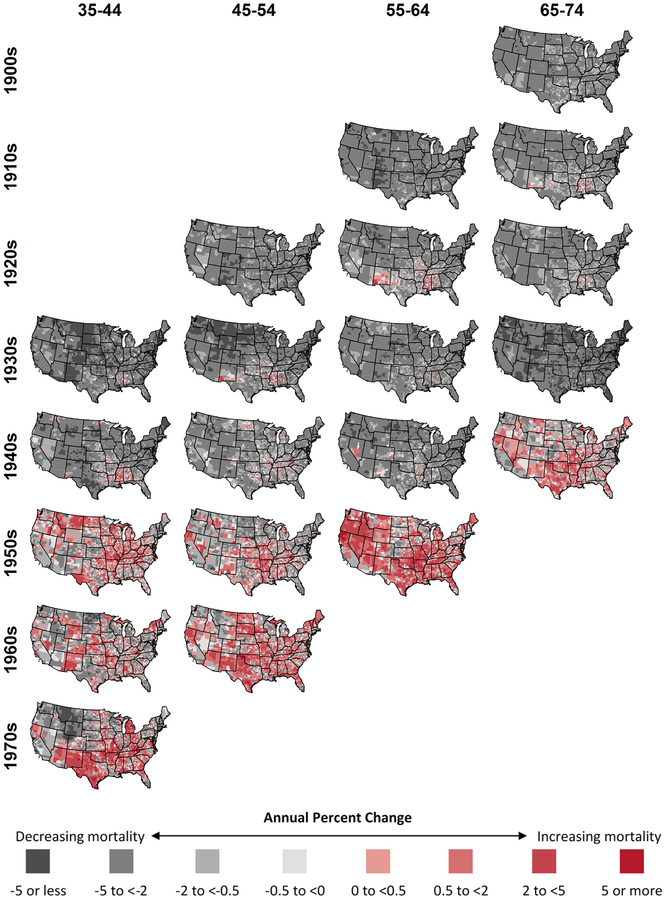
Patterns of changing heart disease death rates within age groups (and across time periods and birth cohorts) are indicated by looking down the columns in Table 1 and Fig. 4. For ages 35–44, increasing heart disease death rates were rare in 1973–1980 (0.8% of counties) and 1980–1990 (5.2%), but common in the following time periods (49.0% in 1990–2000, 31.6% in 2000–2010, 52.3% in 2010–2015). For ages 45–54, few counties experienced increases prior to 2000, but increases were common after 2000 (30.4% in 2000–2010, 58.5% in 2010–2015). For ages 55–64 and 65–74, increases were rare until the last interval (2010–2015) (69.1% and 42.0% for ages 55–64 and 65–74, respectively).
Fig. 4.
Annual county-level percent change in heart disease mortality by age group (columns), time period (rows), and birth cohort (diagonals), United States, 1973–2015.
3.4. Trends in county-level heart disease death rates within calendar time periods
The experiences of different age groups within a given calendar period are indicated by looking across the rows in Table 1 and Fig. 4. From 1973 through 1990, all four age groups had few counties experiencing increased heart disease death rates (Table 1). Counties with increases showed similar geographic patterns in 1973–1980 and 1980–1990 for all age groups and were primarily concentrated in Mississippi, Alabama, Missouri, and New Mexico (Fig. 4). However, beginning in the 1990s, the patterns began to differ across age groups. In 1990–2000, almost half of counties (49.0%) for ages 35–44 were increasing, while all other age groups continued to experience broad decreases. In 2000–2010, while widespread increases in death rates for ages 35–44 continued, heart disease death rates for ages 45–54 also began to exhibit widespread increases (30.4% of counties). By the end of the study period (2010–2015), all four age groups showed widespread increases in heart disease death rates. In this time period, ages 65–74 exhibited the smallest proportion of counties with increasing heart disease death rates (42.0%) and the smallest magnitudes of increases (median of −0.3% and interquartile range (IQR) of −1.4 to 0.8).
3.5. Trends in county-level heart disease death rates within birth cohorts
The life course experience of each approximate birth cohort can be observed by looking down the diagonals in Table 1 and Fig. 4 and at the rows in Fig. 5. Birth cohorts from the 1900s through the 1930s experienced consistently decreasing heart disease death rates in most counties, with very few counties experiencing increases for the duration of the period of observation. Similarly, most counties experienced decreases for the 1940s birth cohort, with the exception of 2010–2015, when the increases were concentrated in the south-central United States. The life course experience of the 1940s birth cohort differs from prior cohorts (those born in the 1930s and earlier) who saw declines across their life course and from subsequent cohort (those born in the 1950s and later) who saw increases across their life course. Counties with decreasing rates were the exception for the birth cohorts in the 1950s, 1960s, and 1970s, when increases were common for all age groups and years and were geographically widespread. Increases in the 1950s birth cohort, which began in the 1990s, were initially concentrated in the Midwest before moving to the rest of the country.
Fig. 5.
Annual county-level percent change in heart disease mortality by age group (columns) and birth cohorts (rows), United States, 1973–2015.
4. Discussion
In this analysis, we examined county-level trends in age group-specific heart disease death rates from 1973 through 2015 over short calendar time periods. Prior to 1990, all age groups generally experienced similar declines, and increases were confined to similar parts of the country. However, after 1990, age-group specific county-level trends started to diverge, with marked increases in 35–44 year olds. By 2010–2015, all age groups were increasing in large numbers of counties. These counties were broadly distributed across the country, and not confined only to those with high heart disease death rates.
By considering the consistency of trends by birth cohorts, differences in the timing of national plateaus in heart disease mortality across age groups were clarified. Birth years from the 1900s through the 1930s showed consistent declines across all age groups and time periods in almost all counties. Birth years in the 1940s showed increases in a large proportion of counties only in the most recent time interval (2010–2015); birth years from the 1950s through the 1970s showed increasing heart disease death rates for all time periods and age groups in large proportions of counties. Previous work has shown strong cohort effects in national heart disease mortality through 1999 and 2010, with increased mortality beginning at the national level in those born in the late 1950s (Kramer et al., 2014; Pearson-Stuttard et al., 2016; Yang, 2008). Our observed county-level variation suggests that in many counties individuals born in the 1940s have also experienced increased heart disease mortality, which has been masked in the observed national increases.
In the absence of local, aggregate risk factor data from across the life course, results from our analytic method lend support to hypothesized drivers of changing heart disease death rates (Ford and Capewell, 2007; Harper et al., 2011; Lloyd-Jones, 2016; Marmot, 2018; Olshansky et al., 2005; Rodgers et al., 2018; Sidney et al., 2016; Zajacova and Montez, 2017). The observed patterns across both age and birth cohorts suggest that both proximal risk factors and social determinants may have driven changes in heart disease death rates at different times. First, the consistency of trends in county-level changes in heart disease mortality that we observed within birth cohorts across age groups support the hypothesized role of worsening life course proximal risk factors (e.g. obesity, diabetes) in recent increases in heart disease death rates (Ford and Capewell, 2007; Lloyd-Jones, 2016; Olshansky et al., 2005; Sidney et al., 2016). This hypothesis is further supported by the differences between birth cohorts in terms of the observed heart disease mortality trends and in the previously documented timing and duration of exposure to the obesity and diabetes epidemics. Nationally, the prevalence of obesity began to increase in the late 1970s and, roughly 15 years later in the early 1990s, the prevalence and incidence of diabetes also began to increase (Geiss et al., 2014; Wang and Beydoun, 2007). Those who died recently (between 2010 and 2015) between ages 45–64 were in early adulthood at the beginning of increases in obesity prevalence and were middle-aged when diabetes prevalence began to increase. Those who died at ages 35–44 during the recent increases of 2010–2015 lived their entire lives coincident to increasing national obesity prevalence and their entire adult lives coincident to increasing national diabetes prevalence (Geiss et al., 2014; Wang and Beydoun, 2007). Our use of county-level data supports this circumstantial evidence and provides additional evidence that would be missing from a national analysis. Widespread increases in heart disease death rates began in the 1990s in ages 35–44 and were concentrated in the Midwest. Similarly, obesity prevalence was highest and had begun to increase during this same time period and in the same region (Hearne et al., 2004; Segal et al., 2017).
However, birth cohort differences in proximal risk factors may not fully explain either the national slowing of heart disease mortality or cardiovascular disease burden (Preston et al., 2018; Roth et al., 2018) or the consistency of age group-specific trends within calendar time periods. Our observed changes in the heart disease mortality trends represent fundamental shifts in the distributions of heart disease and its proximal risk factors. As such, other macro-level forces acting across the population must be considered (Rodgers et al., 2018; Rose, 1985). In our study, the common patterns across age groups within the two earliest time periods and the single most recent time period reinforce the importance of place-based macro-level factors. Early in the study period, the few counties that experienced increases were concentrated in a similar set of counties in the Deep South across age groups. Likewise, the widespread, common experience of increasing heart disease death rates across age groups from 2010 through 2015, which includes the interruption of declines for those born in the 1940s, may illustrate potential effects of recent economic or dietary changes that impact Americans across the country, independent of geography (Chetty et al., 2016; Gebreab et al., 2015; Harper et al., 2011; Rodgers et al., 2018; Sloop et al., 2018; Strumpf et al., 2017).
With the recent local increases in heart disease death rates and insight into historical differences by birth year, calendar time period, and place, continued monitoring of local trends is vital. In addition to reporting data by age group, explicitly reporting mortality and other data (e.g. diabetes and obesity prevalence) by birth years may help public health practitioners and researchers more directly document, examine, and understand these trends, rather than indirectly relying on combinations of age groups and years of death. Finally, these findings suggest the need for enhanced primary and secondary prevention at younger ages, with a particular focus on life course risk factors. Given the widespread nature of recent increases in heart disease death rates, these efforts should not be geographically restricted to places with already high heart disease death rates.
A key strength of this study is its application of a fully Bayesian spatiotemporal model to national surveillance data. By borrowing statistical strength across adjacent counties and years, this model estimates rates that are more precise than other statistical methods, even in counties with small numbers of deaths (Vaughan et al., 2015), thereby permitting the inclusion of all counties. Additionally, this analysis used national vital statistics data that includes all recorded deaths. Finally, by using Poisson regression within the Bayesian results, our estimates of percent change account for imprecision in the heart disease death rates for all included years.
A limitation of this study is our definition of birth cohorts. In a traditional age-period-cohort analysis, the period is a single year, allowing for the calculation of unique, non-overlapping age groups and birth cohorts (Kramer et al., 2014). However, since this analysis was concerned with changes over time, either age groups or birth cohorts were algebraically required to overlap. Since surveillance data are typically reported by age groups, we chose to use non-overlapping age groups, which then necessitated overlapping birth cohorts. Despite this, the overlap is limited (Fig. 1). An additional limitation is that the underlying cause of death reported on death certificates may be misclassified. However, their use for surveillance at the aggregate level is valid (Coady et al., 2001). Local variation in reporting heart disease as the underlying cause of death represents a potential source of bias, but using the broad ICD category (diseases of the heart) rather than subgroups or specific diagnoses reduces potential misclassification (Coady et al., 2001; Ives et al., 2009).
In summary, this analysis of historical and recent trends in county-level heart disease death rates revealed variation in the trends across age groups and across counties, but relative consistency in the trends by birth cohorts. These findings are consistent with the hypothesis that recent increases stem from the beginnings of the obesity and diabetes epidemics. Additionally, the similarity of geographic patterns in both historical and recent trends in heart disease death rates across age groups, which were not observed in prior time periods or uniformly across birth cohorts, provide insight into the potential role of macro-level factors in driving these changes.
Acknowledgments
Sources of financial support
The US Centers for Disease Control and Prevention supported this study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
References
- Anderson RN, Miniño A, Hoyert DL, Rosenberg HM, 2001. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl. Vital Stat. Rep 49, 1–32. [PubMed] [Google Scholar]
- Casper M, Kramer MR, Quick H, Schieb LJ, Vaughan AS, Greer S, 2016. Changes in the geographic patterns of heart disease mortality in the United States 1973 to 2010. Circulation 133, 1171–1180. 10.1161/CIRCULATIONAHA.115.018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, Bergeron A, Cutler D, 2016. The association between income and life expectancy in the United States, 2001–2014. J. Am. Med. Assoc 315, 1750–1766. 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coady SA, Sorlie PD, Cooper LS, Folsom AR, Rosamond WD, Conwill DE, 2001. Validation of death certificate diagnosis for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J. Clin. Epidemiol 54, 40–50. 10.1016/S0895-4356(00)00272-9. [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Patel SA, Beckles GL, Geiss LS, Mehta N, Xie H, Imperatore G, 2017. County-level contextual factors associated with diabetes incidence in the United States. Ann. Epidemiol 28 20–25.e2. 10.1016/j.annepidem.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan BM, Zhao Y, Axon RN, 2010. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. J. Am. Med. Assoc 303, 2043–2050. 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL, 2012. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307, 491 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Ford ES, Capewell S, 2007. Coronary heart disease mortality among young adults in the U.S. From 1980 through 2002. Concealed leveling of mortality rates. J. Am. Coll. Cardiol 50, 2128–2132. 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Mokdad AH, 2004. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care 27, 2444–2449. 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- Ford ES, Mokdad AH, Giles WH, 2003. Trends in waist circumference among U.S. Adults. Obes. Res 11, 1223–1231. 10.1038/oby.2003.168. [DOI] [PubMed] [Google Scholar]
- Gebreab SY, Davis SK, Symanzik J, Mensah GA, Gibbons GH, Diez-Roux AV, 2015. Geographic variations in cardiovascular health in the United States: contributions of state- and individual-level factors. J. Am. Heart Assoc. 4, e001673 10.1161/JAHA.114.001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW, 2014. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. J. Am. Med. Assoc 312, 1218–1226. 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- Gelfand AE, Vounatsou P, 2003. Proper multivariate conditional autoregressive models for spatial data analysis. Biostatistics 4, 11–15. 10.1093/biostatistics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL, 2017. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief, Hyattsville, MD. [PubMed] [Google Scholar]
- Harper S, Lynch J, Smith GD, 2011. Social determinants and the decline of cardiovascular diseases: understanding the links. Annu. Rev. Publ. Health 32, 39–69. 10.1146/annurev-publhealth-031210-101234. [DOI] [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer H-M, Byrd-Holt DD, 1998. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21, 518–524. 10.4158/EP.12.4.358. [DOI] [PubMed] [Google Scholar]
- Hearne SA, Segal LM, Unruh PJ, Earls MJ, Smolarcik P, 2004. F as in Fat: How Obesity Policies Are Failing in America. Washington, DC. [Google Scholar]
- Imes CC, Burke LE, 2014. The obesity epidemic: the USA as a cautionary tale for the rest of the world. Curr. Epidemiol. Rep 1, 82–88. 10.1007/s40471-014-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives DG, Samuel P, Psaty BM, Kuller LH, 2009. Agreement between nosologist and cardiovascular health study review of deaths: implications of coding differences. J. Am. Geriatr. Soc 57, 133–139. 10.1111/j.1532-5415.2008.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba AJ, Scott J, 1980. Estimates of selected comparability ratios based on dual coding of 1976 death certificates by the eighth and ninth revisions of the international classification of diseases. Mon. Vital Stat. Rep 28, 1–19. [Google Scholar]
- Kramer MR, Valderrama AL, Casper ML, 2014. Decomposing black-white disparities in heart disease mortality in the United States, 1973–2010: an age-period-cohort analysis. Am. J. Epidemiol 182, 302–312. 10.1093/aje/kwv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones DM, 2016. Slowing progress in cardiovascular mortality rates: you reap what you sow. JAMA Cardiol. 1, 29–30. 10.1001/jamacardio.2016.1348.Conflict. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PWF, Wolf PA, Levy D, 2006. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 113, 791–798. 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- Mainous AG, Baker R, Koopman RJ, Saxena S, Diaz VA, Everett CJ, Majeed A, 2007. Impact of the population at risk of diabetes on projections of diabetes burden in the United States: an epidemic on the way. Diabetologia 50, 934–940. 10.1007/s00125-006-0528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, 2018. Social causes of the slowdown in health improvement. J. Epidemiol. Community Health 0 10.1136/jech-2018-210580. [DOI] [PubMed]
- Mitchell N, Catenacci V, Wyatt H, Hill JO, 2011. Obesity: overview of an epidemic. Psychiatr. Clin. North Am 34, 717–732. 10.1016/j.psc.2011.08.005.OBESITY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics, 2016. US Census populations with bridged race categories. Published June 23, 2016. [WWW Document]. www.cdc.gov/nchs/nvss/bridged_race.htm accessed 1.10.17. [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS, 2005. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med 352, 1138–1145. 10.1097/01.sa.0000172529.74325.30. [DOI] [PubMed] [Google Scholar]
- Patel SA, Ali MK, Narayan KMV, Mehta NK, 2016. County-level variation in cardiovascular disease mortality in the United States in 2009–2013: comparative assessment of contributing factors. Am. J. Epidemiol 184, 933–942. 10.1093/aje/kww081. [DOI] [PubMed] [Google Scholar]
- Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, Rehm CD, Afshin A, Danaei G, Kypridemos C, Gaziano T, Mozaffarian D, Capewell S, O’Flaherty M, 2016. Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities. Circulation 133, 967–978. 10.1161/CIRCULATIONAHA.115.019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SH, Vierboom YC, Stokes A, 2018. The role of obesity in exceptionally slow US mortality improvement. Proc. Natl. Acad. Sci 201716802. 10.1073/pnas.1716802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick H, Waller LA, Casper M, 2018. A multivariate space-time model for analysing county level heart disease death rates by race and sex. J. R. Stat. Soc. Ser. C (Appl. Stat.) 67, 291–304. 10.1111/rssc.12215. [DOI] [Google Scholar]
- Quick H, Waller LA, Casper M, 2017. Multivariate spatiotemporal modeling of agespecific stroke mortality. Ann. Appl. Stat 11, 2165–2177. [Google Scholar]
- Reilly JJ, Kelly J, 2011. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int. J. Obes 35, 891–898. 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- Reither EN, Hauser RM, Yang Y, 2009. Do birth cohorts matter? Age-period-cohort analyses of the obesity epidemic in the United States. Soc. Sci. Med 69, 1439–1448. 10.1016/j.socscimed.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers A, Woodward A, Swinburn B, Dietz WH, 2018. Prevalence trends tell us what did not precipitate the US obesity epidemic. Lancet Public Health 3, e162–e163. 10.1016/S2468-2667(18)30021-5. [DOI] [PubMed] [Google Scholar]
- Rose G, 1985. Sick individuals and sick populations. Int. J. Epidemiol 14, 32–38. 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- Roth GA, Johnson CO, Abate KH, Abd-Allah F, Ahmed M, Alam K, Alam T, Alvis-Guzman N, Ansari H, Ärnlöv J, Atey TM, Awasthi A, Awoke T, Barac A, Bärnighausen T, Bedi N, Bennett D, Bensenor I, Biadgilign S, Castañeda-Orjuela C, Catalá-López F, Davletov K, Dharmaratne S, Ding EL, Dubey M, Faraon EJA, Farid T, Farvid MS, Feigin V, Fernandes J, Frostad J, Gebru A, Geleijnse JM, Gona PN, Griswold M, Hailu GB, Hankey GJ, Hassen HY, Havmoeller R, Hay S, Heckbert SR, Irvine CMS, James SL, Jara D, Kasaeian A, Khan AR, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Lal D, Larsson A, Linn S, Lotufo PA, Magdy Abd El Razek H, Mazidi M, Meier T, Mendoza W, Mensah GA, Meretoja A, Mezgebe HB, Mirrakhimov E, Mohammed S, Moran AE, Nguyen G, Nguyen M, Ong KL, Owolabi M, Pletcher M, Pourmalek F, Purcell CA, Qorbani M, Rahman M, Rai RK, Ram U, Reitsma MB, Renzaho AMN, Rios-Blancas MJ, Safiri S, Salomon JA, Sartorius B, Sepanlou SG, Shaikh MA, Silva D, Stranges S, Tabarés-Seisdedos R, Tadele Atnafu N, Thakur JS, Topor-Madry R, Truelsen T, Tuzcu EM, Tyrovolas S, Ukwaja KN, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Weintraub R, Wolfe C, Workicho A, Xu G, Yadgir S, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Zaidi Z, Zaki MES, Zipkin B, Afshin A, Gakidou E, Lim SS, Mokdad AH, Naghavi M, Vos T, Murray CJL, 2018. The burden of cardiovascular diseases among US states, 1990–2016. JAMA Cardiol. 98121, 1–15. 10.1001/jamacardio.2018.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal LM, Rayburn J, Beck SE, 2017. The State of Obesity 2017: Better Policies for a Healthier America. Washington, DC. [Google Scholar]
- Sidney S, Quesenberry CP Jr., Jaffe MG, Sorel M, Nguyen-huynh MN, Kushi LH, Go AS, Rana JS, 2016. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 94612, E1–E6. 10.1001/jamacardio.2016.1326. [DOI] [PubMed] [Google Scholar]
- Sloop GD, Weidman JJ, St Cyr JA, 2018. Perspective: interesterified triglycerides, the recent increase in deaths from heart disease, and elevated blood viscosity. Ther. Adv. Cardiovasc. Dis 12, 23–28. 10.1177/1753944717745507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf EC, Charters TJ, Harper S, Nandi A, 2017. Did the Great Recession affect mortality rates in the metropolitan United States? Effects on mortality by age, gender and cause of death. Soc. Sci. Med 189, 11–16. 10.1016/j.socscimed.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, Ben-Ami Shor D, Tzur D, Afek A, Shamiss A, Haklai Z, Kark JD, 2016. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med 374, 2430–2440. 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- Vaughan AS, Kramer MR, Waller LA, Schieb LJ, Greer S, Casper M, 2015. Comparing methods of measuring geographic patterns in temporal trends: an application to county-level heart disease mortality in the United States, 1973 to 2010. Ann. Epidemiol 25, 329–335. 10.1016/j.annepidem.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AS, Ritchey MD, Hannan J, Kramer MR, Casper M, 2017. Widespread recent increases in county-level heart disease mortality across age groups. Ann. Epidemiol 27, 796–800. 10.1016/j.annepidem.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller LA, Carlin B, Xia H, Gelfand AE, 1997. Hierarchical spatio-temporal mapping of disease rates. J. Am. Stat. Assoc 92, 607–617. [Google Scholar]
- Wang Y, Beydoun MA, 2007. The obesity epidemic in the United States-gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol. Rev 29, 6–28. 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V, 2015. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation 132, 997–1002. 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, 2008. Trends in U.S. Adult chronic disease mortality, 1960–1999: age, period, and cohort variations. Demography 45, 387–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova A, Montez JK, 2017. Macro-level perspective to reverse recent mortality increases. Lancet 6736, 1–2. 10.1016/S0140-6736(17)30186-1. [DOI] [PubMed] [Google Scholar]