Abstract
While protein kinases are key components in multiple cellular processes, efficient identification of cognate in vivo substrates remains challenging. Here we describe a powerful method to screen potential substrates of protein kinases by partial transfer of proteins from a 2D-PAGE gel to a Western blot membrane. This approach allowed precise pinpointing of candidate substrate spots in the 2D gel, and identifying physiological substrates of protein kinases in Mycobacterium tuberculosis.
Keywords: modified immunoblot method, substrates of kinases, partial transfer of proteins
Reversible protein phosphorylation is a ubiquitous and essential mechanism that mediates signal transduction pathways of cellular processes in both eukaryotes and prokaryotes. While bacteria generally transduce extracellular signals into cellular responses via two-component systems, they also possess eukaryotic-like protein kinases, though the most widely distributed are Ser/Thr protein kinases (Magasanik, 1995; Leonard et al., 1998). These protein kinases dynamically phosphorylate numerous proteins to control complex signaling network in the cell. Comprehensive screening of substrates for kinases is thus necessary to achieve understanding of the signaling networks in which protein kinases participate. However, it remains a challenge to unambiguously identify the physiological substrates of each protein kinase.
In vitro kinase assays with cell extracts incubated with a protein kinase and [γ−32P]ATP have been used to identify prospective substrates for many years (Ptacek et al., 2005; Villarino et al., 2005; Cohen and Knebel, 2006). However, this approach can lead to non-specific phosphorylation due to non-physiologic concentrations of the kinase of interest and the presence of other protein kinases and their substrates in cell extracts. More recently, genome-wide screening of substrates using protein microarrays, liquid chromatography tandem mass spectrometry combined with phospho-peptide enrichment, and two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) have been used to identify substrates of various kinases (Ptacek et al., 2005; Schmelzle and White, 2006; Machida et al., 2007). Even with these methods, it has been difficult to identify substrates that are weakly phosphorylated and to identify cognate kinase-substrate pairs.
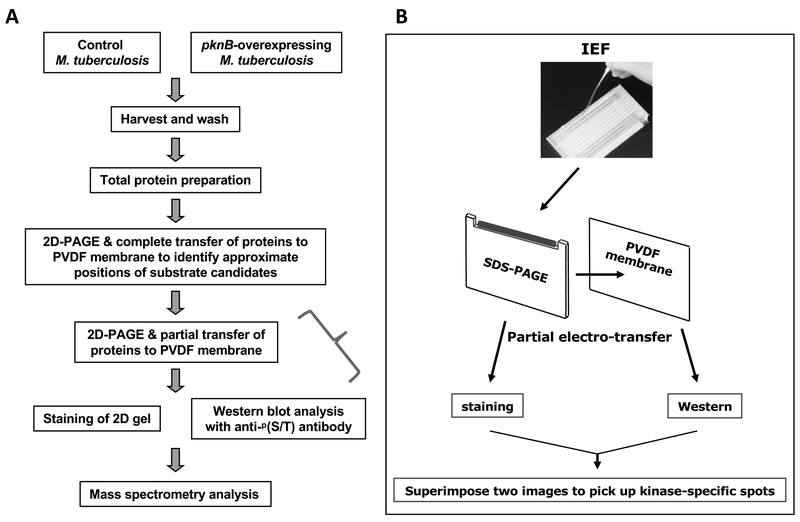
To overcome these limitations, we developed a proteomic approach that involves a partial transfer of proteins from a 2D-PAGE gel to a Western blot membrane to accurately pinpoint the substrate candidates for mass spectrometry. Fig. 1 shows a schematic diagram of our approach to identify cellular substrates of the PknB (a model for protein kinases in this approach) from Mycobacterium tuberculosis. Herein, we describe the partial transfer of proteins into a PVDF membrane in detail. In our study, we overexpressed PknB since it was thought to be essential (Sassetti et al., 2003). Construction and culturing of M. tuberculosis that expresses pknB were performed as previously described (Kang et al., 2005). However, if a deletion mutant of the kinase of interest is available, one can compare the deletion mutant and wild-type cells for this analysis. M. tuberculosis cells harboring a vector alone, or an inducible pknB-overexpression construct, were grown to late log phase, followed by induction of kinase expression (Fig. 1a). Total mycobacterial protein was purified and cleaned with the ReadyPrep 2D Cleanup Kit (Bio-Rad), followed by rehydration into isoelectric focusing (IEF; Lee et al., 1991) strips with a pH range of 3–10 (Bio-Rad, Hercules, USA). IEF was performed in a PROTEAN IEF Cell (Bio-Rad), and second-dimension SDS-PAGE was performed using 10% Tris-HCl Criterion gels (Bio-Rad). Proteins were electro-transferred completely to a PVDF membrane for 60 min at 15 volts, followed by Western blot analysis with a phospho-(S/T)Q polyclonal antibody (Cell Signaling Technology, Boston, USA) since our previous peptide library screening analysis suggested that PknB preferentially phosphorylated Thr adjacent to Gln (Kang et al., 2005). Five spots were found to be clearly stronger in an immunoblot from pknB-overexpressing cells than control cells with vector alone (data not shown), which was identical to our previously described data (Kang et al., 2005).
Fig. 1.
Schematic diagram of the identification of kinase substrates through partial transfer of proteins from 2D-PAGE. (a) Summary flow chart of procedures. (b) IEF and partial transfer of proteins from a 2D-PAGE gel to a PVDF membrane. To pinpoint the candidate substrate protein, images from the Western blot and the stained gel were superimposed.
It is technically difficult to identify a single protein target based on Western blot signals from a 2D-PAGE gel by simply running two gels simultaneously (one for Western blot and the other for staining) because protein spot patterns from the two independent gels often are not identical and proteins of interest can be crowded by many other proteins in 2D gels even if micro-range IEF strips are used. To overcome these problems, we ran one 2D-PAGE gel with a microrange IEF strip (pH=4.7–5.9), proteins were partially transferred to a PVDF membrane which was subsequently used for Western blot with a phospho-(S/T)Q antibody, and the residual proteins in the gel were stained. By superimposing the two images of Western blot and stained gel, we could pinpoint the protein spots that showed positive signal from Western blot (Fig. 1b and Fig. 2). We have used two electro-transfer systems for partial transfer (both from Bio-Rad): Trans-Blot Cell (buffer tank style, Fig. 2) and Trans-Blot SD Semi-dry Transfer Cell (data not shown). The condition for partial transfer of the protein of interest in Fig. 2 (white circle, approximate MW = 30 kDa) was for 15 min at 15 volts for both transfer systems.
Fig. 2.
Pinpoint of a protein spot that matches with Western signal by partial transfer of proteins in 2D-PAGE. Internal marks (Δ) were made on both gel and PVDF membrane after the partial transfer of proteins from 2D-PAGE, before the gel and membrane were separated. The membrane was probed with a phospho-(S/T)Q antibody and the residual proteins in the gel were stained with SYPRO-Ruby. The images from Western blot and gel staining were superimposed and aligned using the internal marks to pinpoint the protein spot that corresponds to the Western spot (white circles).
After the partial transfer, the sandwich of the gel and membrane was carefully removed from the transfer system and position marks were made by cutting out both the gel and membrane with a razor blade (Fig. 2). The PVDF membrane was then removed and used for an immunoblot blot analysis with a phospho-(S/T)Q antibody. The 2D-PAGE gel containing residual proteins was stained with SYPRO-Ruby (Molecular Probes, Eugene, USA). Images from the immunoblot membrane and stained gel were taken by using Image One software in ChemiDoc XRS system (Bio-Rad), and were superimposed to select one of the protein spots that showed higher Western signals in pknB-overexpressing cells (Fig. 2). To identify this substrate candidate of PknB, the protein spot corresponding to the immunoblot signal (white circle, lower panel, Fig. 2) was excised from the stained gel, subjected to a in-gel trypsin plus chymotrypsin digestion, and analyzed by an liquid chromatography tandem mass spectrometry as previously described (Kang et al., 2005). Wag31 was the protein to which most peptides matched (data not shown), which was identical to our previously described data (Kang et al., 2005).
There are several considerations regarding the general utility of this method. First, the optimal condition for partial transfer of proteins to a Western membrane must be experimentally determined. To partially transfer smaller or bigger proteins than ~30 kDa, one can decrease or increase transfer time by several minutes from the condition we established for Wag31. Second, when there is not enough Western signal from a protein spot of interest after partial transfer, one can decrease time or voltage for partial transfer by several minutes or voltages from the condition one established for one’s interesting protein. Thirdly, it is important to make internal marks before the membrane is removed from the gel after the partial transfer, as shown in Fig. 2, because 2D-PAGE gels slightly change their shape during the subsequent staining step. For example, if 2D-PAGE gel is stained with SYPRO-Ruby, the upper part of the gel expands slightly more than the lower part of the gel. In this case, internal marks can ensure the exact alignment of those two images of Western blot and stained gel. Fourthly, before the partial transfer experiment, the approximate position of the protein spots of interest in 2D-PAGE must be determined by a preliminary experiment with complete transfer of proteins to avoid potential missing of the protein spots of interest by those internal marks. Fifthly, phosphorylation of the newly found substrates must be validated with other methods. For example, the candidate substrate can be tagged and affinity-purified from wild-type cells (without kinase overexpression), and tested for phosphorylation by 2D Western with phospho-specific antibodies or autoradiography. Finally, when there are not enough mass spectrometry signals for the identification of phosphorylation of the newly found substrates, one can run and stain multiple 2D gels to excise the protein spots of interest.
In this report, we have described an efficient method to pinpoint the potential substrates of protein kinases. This method could also be used to search for substrates of protein phosphatases for which specific antibodies are commercially available.
Acknowledgments
This work was supported by research grants from Wayne State University to C.M.K. and also from KORDI in-house program (PE98402) and the Marine & Extreme Genome Research Center Program of Ministry of Land, Transport, and Maritime Affairs, Republic of Korea. R.N.H. acknowledges support from NIH grant RO1AI59702. W.J.J. also acknowledges support from grants in part by the Centenary Plan from the University of South Carolina; a start-up fund from the University of South Carolina; the New Investigator Award from the International Foundation; and the Century II Equipment fund, a new faculty start-up package, and the Research Excellent Fund from Michigan Technological University (WJJ). S.H.L. was supported by the Marine & Extreme Genome Research Center Program of Ministry of Land, Transport, and Maritime Affairs, Republic of Korea and Basic Science Research Program through the National Research Foundation (NRF) of Korea (KRF-2008–313-C00790) funded by the MEST.
References
- Cohen P and Knebel A. 2006. KESTREL: a powerful method for identifying the physiological substrates of protein kinases. Biochem. J 393, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CM, Abbott DW, Park ST, Dascher CC, Cantley LC, and Husson RN. 2005. The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev. 19, 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Pascopella L, Jacobs WR Jr., and Hatfull GF. 1991. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guerin. Proc. Natl. Acad. Sci. USA 88, 3111–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Aravind L, and Koonin EV. 1998. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 8, 1038–1047. [DOI] [PubMed] [Google Scholar]
- Machida M, Kosako H, Shirakabe K, Ushiyama M, Inagawa J, Hirano J, Nakano T, Bando Y, Nishida E, and Hattori S. 2007. Purification of phosphoproteins by immobilized metal affinity chromatography and its application to phosphoproteome analysis. FEBS J. 274, 1576–1587. [DOI] [PubMed] [Google Scholar]
- Magasanik B 1995. Historical perspective, pp. 1–5. In Hoch J and Silhavy T (eds.), Two Component Signal Transduction. ASM Press, New York, Washington, USA. [Google Scholar]
- Ptacek J, Devgan G., Michaud G, Zhu H., Fasolo J, Guo H., Jona G, Beritkreutz A, Sopko R, McCartney RR, Schmidt MC, Rachidi N, Lee SJ, Mah AS, Meng L, Strak MJR, Stern DF, Virgilio CD, Tyers M, Andrews B, Gerstein M, Schweitzer B, Predki PF, and Snyder M. 2005. Global analysis of protein phosphorylation in yeast. Nature 438, 679–684. [DOI] [PubMed] [Google Scholar]
- Sassetti CM, Boyd DH, and Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol 48, 77–84. [DOI] [PubMed] [Google Scholar]
- Schmelzle K and White FM. 2006. Phosphoproteomic approaches to elucidate cellular signaling networks. Curr. Opin. Biotechnol 17, 406–414. [DOI] [PubMed] [Google Scholar]
- Villarino A, Duran R, Wehenkel A., Fernandez P, England P, Brodin P, Cole ST, Zimny-Arndt U, Jungblut PR, Cerveñansky C, and Alzari PM. 2005. Proteomic identification of M. tuberculosis protein kinase substrates: PknB recruits GarA, a FHA domain-containing protein, through activation loop-mediated interactions. J. Mol. Biol 350, 953–963. [DOI] [PubMed] [Google Scholar]