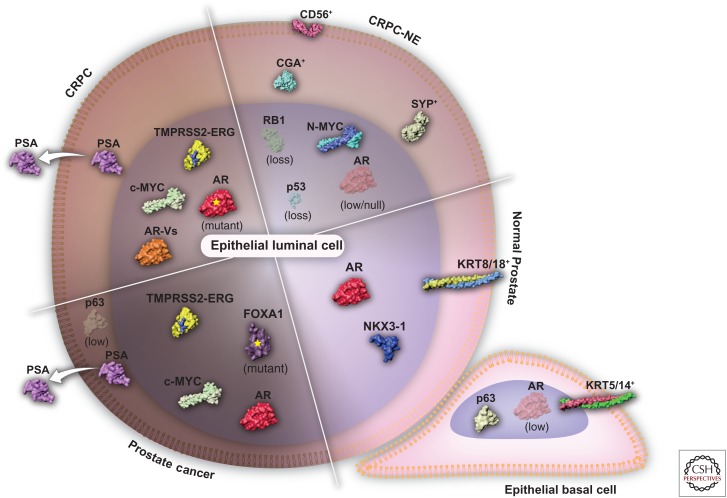
Figure 1.
Evolution of the prostate cancer network of transcription factors throughout disease progression. Normal prostate epithelium consists of basal (KRT5/14+) and luminal cells (KRT8/18+) with a transcriptional network governed by p63 or AR and NKX3-1, respectively. Prostate cancer is characterized by the expansion of AR-dependent epithelial cells harboring genetic alterations such as TMPRSS2-ERG gene fusion, c-MYC amplification, and FOXA1 mutations. The loss of the epithelial basal cell layer and the disruption of the normal tissue architecture results in the secretion of trackable levels prostate-specific antigen (PSA) into the bloodstream. The first line of androgen receptor (AR)-directed therapy results in the emergence of a castration-resistant prostate cancer (CRPC) through AR amplification, mutation, or the formation of AR messenger RNA (mRNA) splice variants (AR-Vs). Castration-resistant neuroendocrine prostate cancer (CRPC-NE) emerges following the resistance to the second generation of AR-targeted therapies. In contrast to the other stages of the disease, CRPC-NE (SYP+/CGA+/CD56+) is AR independent and is often driven by MYCN amplification (encoding N-MYC) and the loss of RB1 and TP53 (encoding p53) tumor-suppressing genes.