Abstract
Aims:
This study was designed to determine specific cell groups of the raphe nuclei (RN) that give rise to supraspinal serotonergic projections regulating the bladder reflex.
Methods:
Anesthetized rats underwent surgery to open the abdomen and expose the bladder. A total of 6 μL transsynaptic neuronal tracer pseudorabies virus (PRV-152), encoding for green fluorescent protein (GFP), was injected into the bladder detrusor. After 72 or 96 h, animals were perfused and the brain was dissected for processing transverse and sagittal sections. Subsequently, fluorescent immunohistochemistry for GFP and Serotonin (5-hydroxytryptamine [5-HT]) was performed in the brain sections. Under the microscope, each RN subset was characterized individually from caudal to rostral according to the atlas. GFP+ or GFP/5-HT double labeled neurons in each subset were quantified for statistical analysis.
Results:
At 72-h post-infection, very few GFP+ or GFP/5-HT double-labeled neurons appeared in the brainstem and beyond. In contrast, many labeled neurons were found at these levels after 96 h. Quantitative analysis showed that the majority of infected 5-HT+ neurons were located in the pallidus, obscurus, and magnus nuclei. Conversely, very few infected neurons were found in other raphe subsets, that is the pontis, median, dorsal, or linear nuclei. Overall, the raphe magnus had the highest number of GFP-labeled and GFP/5-HT double-labeled cells.
Conclusions:
The caudal subsets of RN, especially the raphe magnus, are the main sources of serotonergic input to the lower spinal cord controlling bladder activity.
Keywords: detrusor, pseudorabies virus, retrograde tracing, serotonin, urinary
1 |. INTRODUCTION
The lower urinary tract (LUT) has two different functions, storage and excretion of urine. Micturition behavior is reciprocally controlled by autonomic and somatic nervous systems.1 In autonomic control, the sympathetic pathway supports continence by inhibiting contraction of the detrusor when there are lower volumes of urine within the bladder. Once the intravesical pressure of the bladder reaches a threshold, the parasympathetic pathway stimulates the bladder detrusor for contraction to facilitate a void. Cortical and subcortical regulations render a coordinated interaction between autonomic bladder and somatic sphincter muscle activities for the balance of these opposite functionalities.2 During the process of urination, multiple neurotransmitter systems are involved in the supraspinal regulation of urinary function.
Serotonin (5-hydroxytryptamine [5-HT]) is one of the neurotransmitters in the complex micturition neuronal circuitry. It was first introduced by Dahlstrom and Fuxe in the 1960s, based on the observation of dense 5-HT-containing nerve fibers innervating sympathetic and parasympathetic nuclei throughout the spinal cord.3 Ensuing pharmacological studies demonstrated that application of serotonin onto either sympathetic or parasympathetic preganglionic neurons altered their firing and influenced the micturition reflex.4,5 Subsequently, distinct subtypes of 5-HT receptors were characterized not only in supraspinal sites but also in the spinal cord,6 indicating central 5-HT mechanisms involved at multiple levels in the nervous system. Previous studies showed that, in the rat spinal cord, stimulating 5-HT1A receptors had an excitatory physiological role in the control of bladder function whereas activation of 5-HT2A receptors inhibited the reflex.7,8 Thus, serotonin is an important contributor to the modulation of LUT function along with other neurotransmitters.
It has been known that the caudal raphe nuclei (RN) are the main sources of supraspinal serotonergic projections. The RN are 5-HT neuron-enriched cell clusters located in the brainstem, pons, and midbrain, which include from the caudal to rostral, the nucleus raphe pallidus (RPa, B1), the nucleus raphe obscurus (ROb, B2), the nucleus raphe magnus (RMg, B3), the nucleus raphe pontis (RPn, B4), the median raphe nucleus (MnR, B5, and B8), the dorsal raphe nucleus (DR, B6, and B7), and the linear raphe nucleus (LiR, B9).9 Regarding the widely-distributed raphe subsets in the hindbrain, however, the detailed anatomical cell origination of these descending pathways to a specific pelvic organ, for example the bladder, is still unclear. The pseudorabies virus (PRV), a transsynaptic neuronal tracer, has been used to map the LUT neuronal circuitry. The major advantage of PRV is viral transmission from an infected neuron to other neurons connected with synaptic formation, thus defining the signal specificity of connectivity between neurons.10 With different time courses post to inoculation, different classes of neurons involved in the neuronal circuits can be infected, and labeled. Previous studies using this technique to trace the bladder or urethra have successfully shown PRV labeled cells in some of the most prominent brain areas, such as the pontine micturition center (PMC) and the RN.11–13 To further understand the specific organization of supraspinal serotonergic pathways controlling the bladder function, in the present study, we injected PRV-152 into the rat bladder detrusor, and investigated labeled 5-HT neurons in each subsets of RN. This experiment was aimed to answer the following questions: 1) which subsets of RN mainly project serotonergic fibers to the spinal cord and modulate the bladder function and 2) what the extent of other parts of the RN contributes to the descending pathways. The results would fill the anatomical knowledge gap about serotonergic regulation of LUT function, and provide the guidance for the specific manipulation of 5-HT neuronal activity in the control of micturition.
2 |. MATERIALS AND METHODS
2.1 |. Animals
A total of 16 adult female Wistar rats (weight 200–250 g) were used. Institutional Animal Care and Use Committee and Society for Neuroscience guidelines on animal care were strictly followed to minimize potential suffering and the number of animals used.
2.2 |. PRV-152 injection
Animals were anesthetized with 2% isoflurane for surgery. After the abdomen was shaved and cleaned with a Xenodine solution, rats were placed on the surgical station in supine position. A No. 10 blade was used to make a surgical lesion on the lower abdominal wall to expose the bladder. A total of 6 μL of PRV-152 (Bartha strain, 109 pfu/mL, courtesy of Dr. Michael A. Lane), encoding for green fluorescent protein (GFP), was injected into the bladder detrusor at three different sites, including the front, and bilateral sides, 2 μL per site, with a 10 μL Hamilton syringe connected with a fine glass tip.14,15 Injection sites were immediately sealed with a small drop of tissue glue. Overlying musculature and skin were then closed. Animals were administered with cefazolin (10 mg/kg) and buprenex (0.1 mg/kg; Reckitt Benckiser, Slough, United Kingdom) post-operation.
2.3 |. Histology
Animals survived for an additional 72 (n = 5) or 96 h (n = 11) after PRV-152 injection.11 Rats were euthanized with an overdose of Euthasol and then transcardially perfused with 200 mL ice-cold 0.1 M phosphate buffer saline (PBS), pH 7.4, followed by 4% paraformaldehyde (PFA) in PBS. The brain was dissected and post-fixed in 4% PFA and subsequently cryoprotected through infiltration in 30% sucrose at 4°C overnight. The samples were then embedded with optimum cutting temperature (OCT) compound on top of dry ice. Transverse (n = 5 in 72 h, n = 7 in 96 h) or sagittal (n = 4 in 96 h) sections were obtained in 35 μm using a cryostat. Freefloating sections were collected as six serials in order and kept in the solution, containing 25% glycerol and 30% ethylene glycol in 0.1 M phosphatase buffer, at −20°C. Thus, one serial of samples included tissue sections were separated with 210 μm apart.
One serial free-floating transverse or sagittal tissue section was used for immunostaining, including approximately 30–35 transverse or 15–20 sagittal ones. Sections were washed three times in Tris-buffered saline (TBS), and then incubated in TBS blocking buffer (containing 5% goat serum and 0.5% triton X100) for 1 h at room temperature. Subsequently, tissue sections were incubated in primary antibodies diluted with blocking buffer solution overnight at 4°C. Primary antibodies were against 5-hydroxytyptamine (5-HT, rabbit, 1:1000, Millipore, Burlington, MA) and GFP (mouse, 1:500, Millipore). The sections were rinsed and incubated in goat anti-rabbit IgG conjugated with Alexa 594 and goat anti-mouse IgG conjugated with Alexa 488 secary antibody solution (both 1:500, Invitrogen, Carlsbad, CA) for 3 h minimum at room temperature on a shaker. Sections were then washed three times, mounted on the slide, and coverslipped with 200 μL FluorSave aqueous mounting medium (Millipore).
Immunostained sections were observed and imaged under a fluorescent microscope (Leica DM5500B, Wetzlar, Germany). Cell counting was performed under a 20× objective in one serial transverse sections. The boundaries of the serotonergic nuclei were determined by the relative location of the 5-HT-labeled cells and were carefully estimated with referencing of the rat brain atlas. GFP+, 5-HT+, or GFP/5-HT double-labeled cells in each subset of the RN were counted in each section. Cells outside of the designated nuclei were disregarded and therefore only cells within the nuclei were counted. The total number of GFP+, 5-HT+, or GFP/5-HT double-labeled cells in each subset of the RN were obtained by summing counted cells in one whole serial sections per rat. The quantitative data and the ratio between the GFP+, 5-HT+, and GFP/5-HT double-labeled cells were statistically analyzed.
2.4 |. Statistical analysis
Statistical analyses were performed in IBM SPSS 24. Unpaired Student’s t-test was employed to compare the number of double-labeled cells between 72 and 96 h in each nucleus. The total number of double-labeled cells and the ratio to GFP or 5HT-labeled cells were compared between subsets of the RN. Significance was set at P < 0.05. Data were represented as mean ± SEM.
3 |. RESULTS
In the transsynaptic tracing, PRV-infected neurons in the CNS initially emerged bilaterally in the lumbosacral spinal cord, and then in the brainstem and higher structures with time course. Labeled neurons in the spinal cord were mainly located in the sympathetic/parasympathetic autonomic regions. At 72-h post-infection, very few GFP-labeled neurons appeared in the brainstem and beyond. By contrast, many neurons are labeled at 96-h post-infection, indicating that more time was necessary for the cycle of viral replication within early-labeled neurons and subsequent transportation through synaptic connectivity to the next neurons. The structures containing infected neurons, from the brainstem to hypothalamus, included the caudal RN, the locus ceoruleus (LC), the A5 region, the PMC in the brainstem, the periaquaductal gray (PAG), and the paraventricular nucleus (PVN). The labeling patterns were very similar to previous reports.12
After 72-h viral infection and transportation, double immunostaining for GFP and 5-HT revealed that a few infected serotonergic neurons emerged in the different divisions of RN, mainly the caudal subsets, such as the raphe pallidus, the raphe obscurus, and the raphe magnus. Very few or no GFP-labeled 5-HT neurons were detected in the rostral parts of RN. At 96-h post-infection, however, the number of GFP-labeled 5-HT neurons was dramatically increased in the caudal raphe subsets, especially the raphe magnus. The majority of labeled neurons in the caudal RN exhibited large soma and clear axonal extensions. In addition, a small number of GFP/5-HT double-labeled neurons occurred in the rostral parts of RN, including the raphe pontis, the median raphe, the dorsal raphe, and the linear raphe. Labeled neurons in the rostral RN often displayed small size of cell body. Notably, many GFP-labeled but 5-HT− neurons were present in the caudal RN. Considering the other phenotypes of neurons existing in the RN, this finding suggests that neurons other than serotonergic ones in the RN also project to the spinal cord regulating bladder activity. In reference to the atlas of Paxinos and Watson,16 structural diagrams were outlined to compare the level and location of labeled raphe subsets in the transverse brain section from the rostral to caudal (Figure 1). In sagittal sections, the morphology of each raphe subset was displayed, which was very different from the transverse view. A sagittal diagram combined with immunostained raphe divisions was provided to illustrate the position of distinct raphe subsets (Figure 2).
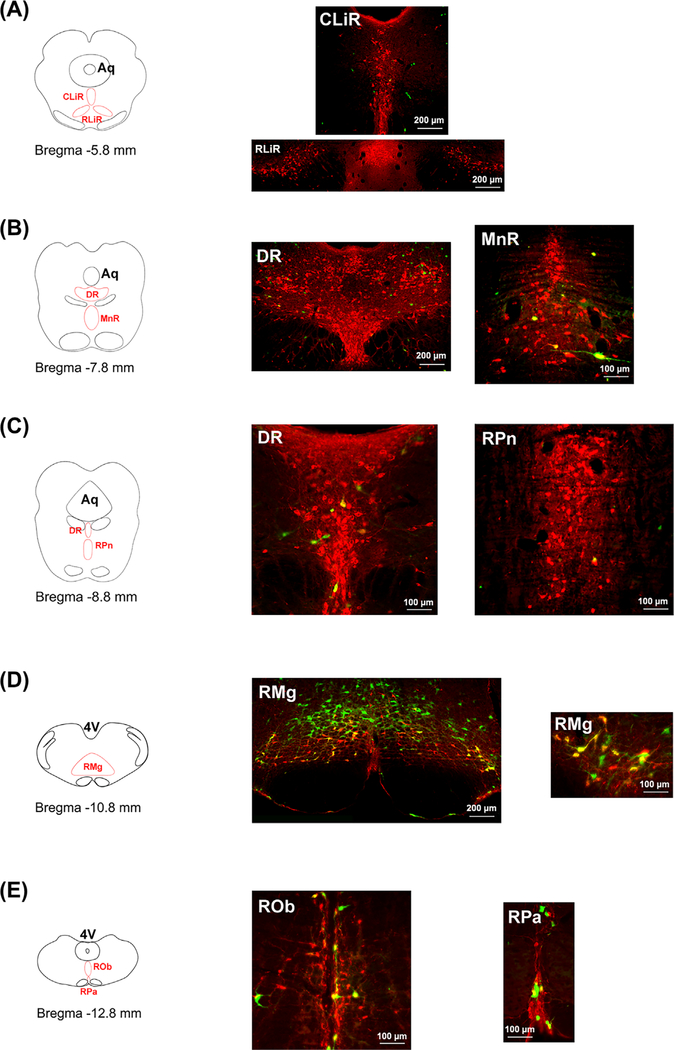
FIGURE 1.
Pseudorabies virus (PRV)-labeled serotonergic neurons in the coronal sections of the brain. At 96 h after injection of PRV into the bladder wall, immunostaining demonstrates that GFP-labeled serotoninergic (5-HT+) neurons are detected in the brainstem and midbrain. A-E, From the rostral to caudal, these double-labeled neurons are shown in each subset of the RN in line with the location of nucleus in the map. The majority of PRV-labeled serotonergic neurons emerge in the medullary parts of raphe nuclei, including the RPa, ROb, and RMg (B1-B3 cell groups). Additionally, some GFP-labeled neurons are 5-HT−, suggesting the involvement of non-serotonergic raphe neurons in bladder control. The small image in D shows higher magnification of GFP/5-HT double labeled cells (RPa, raphe pallidus; ROb, raphe obscurus; RMg, raphe magnus; RPn, raphe pontis; MnR, median raphe; DR, dorsal raphe; CLiR, caudal linear raphe; RLiR, rostral linear raphe; Aq, aquaduct; 4V, the 4th ventricle)
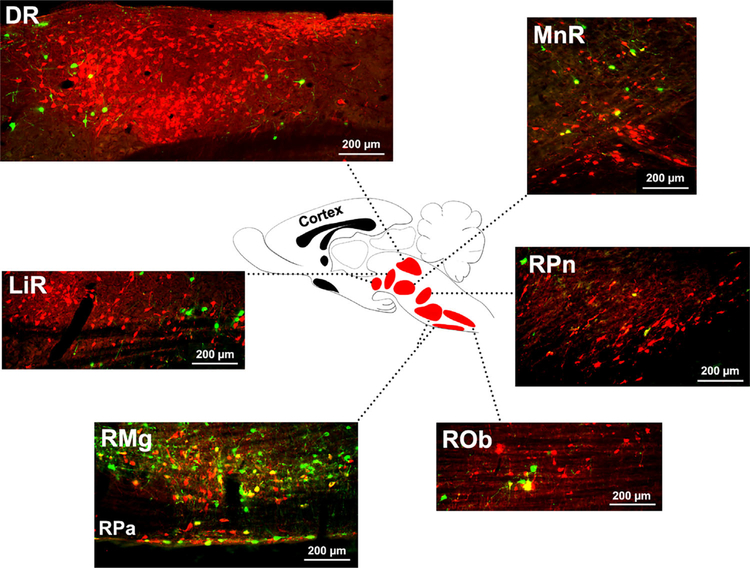
FIGURE 2.
The sagittal view of the raphe nuclei subsets and the distribution of pseudorabies virus (PRV)-labeled serotonergic (5-HT+) cells at 96 h post to infection. Unlike very few GFP-labeled 5-HT+ neurons in the rostral cell groups of raphe nuclei, most double immunolabeled cells are mainly present in the caudal subsets. Understanding the labeling pattern in both transverse and sagittal planes renders better clarification of cell distribution in 3-dimensions. Overall, the result of PRV-tracing suggests a complex network of serotonergic neurons modulating the bladder reflex (RPa, raphe pallidus; ROb, raphe obscurus; RMg, raphe magnus; RPn, raphe pontis; MnR, median raphe; DR, dorsal raphe; LiR, linear raphe)
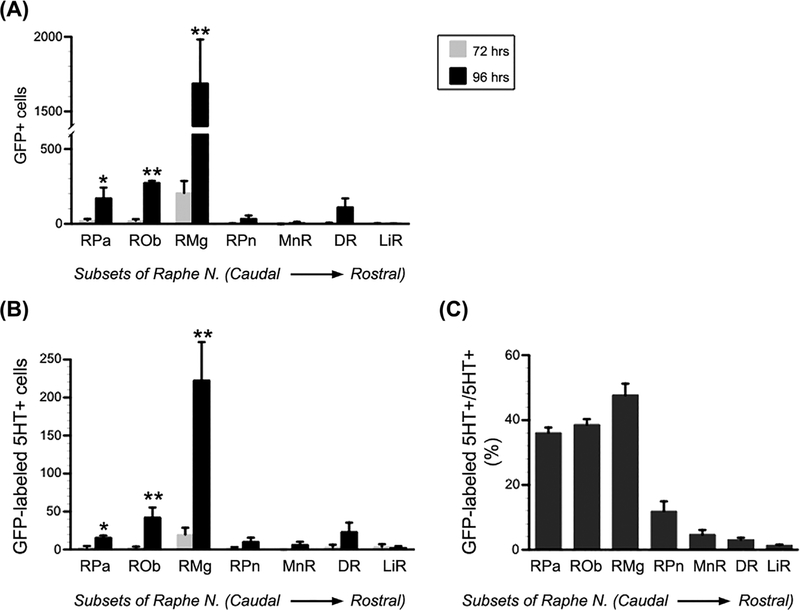
In one serial transverse sections, the total number of GFP+, 5-HT+, or GFP/5-HT double-labeled neurons was counted in each raphe subset. The majority of GFP-labeled or GFP/5-HT double-labeled neurons were recognized in the raphe magnus. Statistical analysis demonstrated that, compared to 72-h labeling, significantly more GFP-labeled or GFP/5-HT double-labeled neurons emerged in the caudal RN at 96 h post-infection (GFP-labeled: RPa 22.6 ± 10.5 vs 173.4 ± 11.1, P < 0.05; Rob 21.2 ± 10.8 vs 275.6 ± 68.7, P < 0.01; RMg 206.8 ± 79.5 vs 1690.6 ± 292.0, P < 0.01; GFP/5-HT double labeled: RPa 2.8 ± 1.9 vs 16.0 ± 2.4, P < 0.05; Rob 2.6 ± 1.3 vs 42.2 ± 12.9, P < 0.01; RMg 19.8 ± 8.8 vs 222.7 ± 50.1, P < 0.01; Unpaired Student’s ttest) (Figures 3A and 3B). Nevertheless, no significant difference was detected in either GFP-labeled or GFP/5-HT double-labeled neurons (all P > 0.05; Student’s t-test) in the rostral raphe subsets between 72 and 96 h post-infection, including the raphe pontis, the dorsal raphe, the median raphe, and the linear raphe. At 96-h post-infection, the ratio of GFP/5-HT double-labeled neurons to GFP-labeled neurons is 11.0 ± 2.5% in the raphe pallidus, 16.0 ± 2.7% in the raphe obscurus, and 15.5 ± 1.6% in the raphe magnus. Remarkably, high percentage of GFP-labeled 5-HT+ to the total of 5-HT+ neurons is detected in the three caudal raphe nuclei, such as 36.1 ± 1.6% in the raphe pallidus, 38.6 ± 1.7% in the raphe obscurus, and 47.7 ± 3.5% in the raphe magnus (Figure 3C). The results disclosed a detailed distribution of infected serotonergic or non-serotonergic neurons in the different raphe parts after PRV inoculation into the bladder. Thus, this finding suggests that a complex network of serotonergic neurons in the brain modulates the micturition function.
FIGURE 3.
GFP-labeled or GFP/serotonin (5-HT) double-labeled neurons are quantified in each subset of the raphe nuclei. A and B, Statistical analysis indicates significantly more (A) GFP-labeled or (B) GFP/5-HT double-labeled neurons in the caudal raphe nuclei at 96-hr post-infection than 72 h, including the RPa, ROb, and RMg. However, very few GFP-labeled or GFP/5-HT double-labeled neurons were detected in the rostral raphe nuclei, that is the RPn, MnR, DR, and LiR, at either 72 or 96 h post-infection. C, High percentage of GFP-labeled 5-HT+ to the total of 5-HT+ neurons is detected in the 3 caudal raphe nuclei (Unpaired Student’s t-test, *P < 0.05, **P < 0.01; RPa, raphe pallidus; ROb, raphe obscurus; RMg, raphe magnus; RPn, raphe pontis; MnR, median raphe, DR, dorsal raphe; LiR, linear raphe)
4 |. DISCUSSION
Transsynaptic tracing demonstrates the specificity of retrograde infection of synaptically connected neurons. With the combination of PRV-152 injection and immunolabeling, we investigated the origin of supraspinal serotonergic projections regulating the bladder activity. PRV-infected 5-HT neurons were mostly found in the caudal raphe subsets, known as B1B3 cell groups. The raphe magnus contained the highest number of infected 5-HT neurons. Conversely, labeled cells occasionally occurred in the rostral raphe subsets. Therefore, the caudal raphe subsets mainly contribute supraspinal serotonergic input controlling the bladder activity.
Since the retrograde transport mechanism is the dominant mode of PRV movement, appropriate viral replication and transportation time is critical for tracing components of neuronal circuits.10 Using similar PRV injection schemes, previous studies reported that labeled neurons appeared in the brain only after 72-h post-inoculation, whereas more extensive labeling emerged in the brain at 96 h.12,14 In the present study, labeling incidence and patterns are consistent with that described before. At 96-h post-infection, a larger number of labeled 5-HT neurons were found in the comparable raphe subsets than those at 72 h, indicating that these caudal raphe nuclei are the major parts exerting serotonergic regulation on the bladder reflex. Although the lower urinary tract system receives autonomic and somatic efferent innervations from the lumbosacral spinal cord, neuronal control of the bladder is only involved in the sympathetic and parasympathetic machineries. In rats, sympathetic preganglionic neurons at L1–2 spinal levels and parasympathetic preganglionic neurons at L6/S1 spinal levels are related to the bladder activity.17 Many neuronal populations in the brainstem, midbrain, and hypothalamus are verified to be engaged in the micturition function as revealed by transsynaptic labeling with PRV injection into the bladder wall, urethra, and/or external urethral sphincter (EUS).11–13,18 Descending axon projections from most of these regions release multiple neurotransmitters in the lumbosacral spinal cord. 5-HT is one of them and modulates the micturition reflex. Its function is considered to be mediated via distinct 5HT receptors. Particularly, administration of 5-HT1A receptor agonists facilitates the void, while the receptor antagonists reverse these effects in intact and chronically spinalized rats.19–21 It is assumed that the supraspinal pathway extend in the bilateral dorsolateral funiculus and finally innervate the intermediolateral cell column (IML), where the axon terminals make synapses with sympathetic or parasympathetic preganglionic neurons. Based on the present results, we now know the detailed origin of the serotonergic descending pathway. Notably, this tracing method can only label a small portion of projects to the autonomic region relevant to bladder control.
Cell quantification disclosed a larger amount of GFPlabeled neurons than GFP/5-HT double labeled neurons in each raphe subset. The main reason for this is that serotonergic neurons are not the only type of cells in the RN. Previous studies have reported a large number of nonserotonergic neurons in the RN. These neurons in the dorsal RN express a variety of neurotransmitters or neuromodulators such as γ-aminobutyric acid (GABA), glutamate, and dopamine.22,23 Additionally, a number of neuropeptides and nitric oxide have been characterized in this nucleus.24 Non-serotonergic cells may play a role in regulation of the activity of 5-HT neurons.25 That is to say, non-serotonergic cells are mainly considered interneurons. Regarding their short distance to 5-HT neurons, these interneurons could be labeled at similar time-point as serotonergic neurons. However, we cannot rule out the possibility that some GFP-labeled non-serotonergic cells project directly to the spinal cord and innervate the target neurons because they emerged simultaneously with 5-HT neurons in the scenario. In addition, another possible reason for high number of GFPlabeled neurons is the inclusion of labeled neurons in the vicinity of RN in consideration of the difficulty to precisely discern the border of raphe subsets. Collectively, GFP-labeled non-serotonergic neurons might come from three sources: 1) interneurons; 2) neurons in the RN directly projecting to the spinal cord; and 3) neurons neighboring the RN.
Understanding the distribution and organization of 5-HT cells involved in the bladder activity is important for further exploring the mechanism of LUT function as well as the dysfunctions that arise in pathological conditions. Now we know the medullary raphe magnus contains most serotonergic neurons controlling the bladder function. To study the precise role of these neurons and the interaction with other neurotransmitters, for instance, one can inject viral vectors into the raphe magnus in combination with genetic tools to specifically infect 5-HT neurons. By employing chemogenetic or optogenetic techniques, we would be able to manipulate the activation of serotonergic neurons to dissect their role in urinary function. Following spinal cord injury, supraspinal pathways modulating the LUT activity, including serotonergic ones, are interrupted. This neuronal circuit may be reestablished through increasing cell regenerative capacity for axon growth and target reinnervation for micturition recovery.
5 |. CONCLUSIONS
Overall, three caudal raphe subsets, such as the raphe pallidus, the raphe obscurus, and the raphe magnus, mainly derive supraspinal serotonergic pathways controlling the bladder activity, while the rostral raphe subsets sporadically elongate the descending projections. Particularly, 5-HT neurons in the raphe magnus contribute to the major supraspinal serotonergic input for micturition regulation.
ACKNOWLEDGMENTS
PRV-152 was produced by Dr David Bloom (University of Florida) and generously provided by Dr Michael A. Lane (Drexel University). We thank Ms. Idiata Iredia for technical help. Support for this work was provided by NIH NINDS R01NS099076 and Morton Cure Paralysis Funds to S. Hou, NIH NINDS R01NS085426 and DoD/CDMRP W81XWH-14–1-0605 to V.J. Tom.
Funding information
NIH NINDS, Grant numbers: R01 NS085426, R01 NS099076; DoD/CDMRP, Grant number: W81XWH-14–1-0605; Morton Cure Paralysis Funds
Footnotes
Karl-Erik Andersson led the peer-review process as the Associate Editor responsible for the paper.
REFERENCES
- 1.Fowler CJ, Griffiths D, de Groat WC. The neural control ofmicturition. Nat Rev Neurosci. 2008;9:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control ofnormal and overactive bladder. J Urol. 2005;174:1862–1867. [DOI] [PubMed] [Google Scholar]
- 3.Dahlstrom A, Fuxe K. Localization of monoamines in the lowerbrain stem. Experientia. 1964;20:398–399. [DOI] [PubMed] [Google Scholar]
- 4.Ryall RW, DeGroat WC. The microelectrophoretic administrationof noradrenaline, 5-hydroxytryptamine, acetylcholine and glycine to sacral parasympathetic preganglionic neurones. Brain Res. 1972;37:345–347. [DOI] [PubMed] [Google Scholar]
- 5.Steers WD, de Groat WC. Effects of m-chlorophenylpiperazine onpenile and bladder function in rats. Am J Physiol. 1989;257: R1441–R1449. [DOI] [PubMed] [Google Scholar]
- 6.Ramage AG. The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br J Pharmacol. 2006;147:S120–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang HY, Cheng CL, Chen JJ, de Groat WC. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Gu B, Wu G, et al. The effect of the 5-HT2A/2C receptoragonist on micturition in rats with chronic spinal cord injury. JUrol. 2013;189:1982–1988. [DOI] [PubMed] [Google Scholar]
- 9.Tork I Anatomy of the serotonergic system. Ann N Y Acad Sci. 1990;600:9–34; discussion 34–35. [DOI] [PubMed] [Google Scholar]
- 10.Card JP, Rinaman L, Schwaber JS, et al. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cordlabeled after pseudorabies virus injected into the external urethral sphincter. J Comp Neurol. 1996;375:502–517. [DOI] [PubMed] [Google Scholar]
- 12.Nadelhaft I, Vera PL, Card JP, Miselis RR. Central nervous systemneurons labelled following the injection of pseudorabies virus into the rat urinary bladder. Neurosci Lett. 1992;143:271–274. [DOI] [PubMed] [Google Scholar]
- 13.Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC.Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol. 1995;355:629–640. [DOI] [PubMed] [Google Scholar]
- 14.Nadelhaft I, Vera PL. Central nervous system neurons infected bypseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995;359: 443–456. [DOI] [PubMed] [Google Scholar]
- 15.Hou S, Carson DM, Wu D, Klaw MC, Houle JD, Tom VJ. Dopamine is produced in the rat spinal cord and regulates micturition reflex after spinal cord injury. Exp Neurol. 2016;285: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson G. The Rat Brain in Stereotaxic Coordinates. Orlando, FL: Academic Press; 1986. [Google Scholar]
- 17.Hou S, Rabchevsky AG. Autonomic consequences of spinal cordinjury. Compr Physiol. 2014;4:1419–1453. [DOI] [PubMed] [Google Scholar]
- 18.Rouzade-Dominguez ML, Miselis R, Valentino RJ. Central representation of bladder and colon revealed by dual transsynaptic tracing in the rat: substrates for pelvic visceral coordination. Eur J Neurosci. 2003;18:3311–3324. [DOI] [PubMed] [Google Scholar]
- 19.Chang HH, Havton LA. Serotonergic 5-HT(1A) receptor agonist(8-OH-DPAT) ameliorates impaired micturition reflexes in a chronic ventral root avulsion model of incomplete cauda equina/ conus medullaris injury. Exp Neurol. 2013;239:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SC, Hsieh TH, Fan WJ, Lai CH, Peng CW. Does pharmacological activation of 5-HT1A receptors improve urine flow rate in female rats? Am J Physiol Renal Physiol. 2016;311: F166–175. [DOI] [PubMed] [Google Scholar]
- 21.Reese J, Xiao Z, Schwen Z, et al. Effects of duloxetine andWAY100635 on pudendal inhibition of bladder overactivity in cats. J. Pharmacol Exp Ther. 2014;349:402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA.Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull. 2007;72:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iceman KE, Harris MB. A group of non-serotonergic cells is CO2stimulated in the medullary raphe. Neuroscience. 2014;259:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu W, Le Maitre E, Fabre V, Bernard JF, David Xu ZQ, Hokfelt T.Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010;518: 3464–3494. [DOI] [PubMed] [Google Scholar]
- 25.Monti JM. The role of dorsal raphe nucleus serotonergic and nonserotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 2010;14: 319–327. [DOI] [PubMed] [Google Scholar]