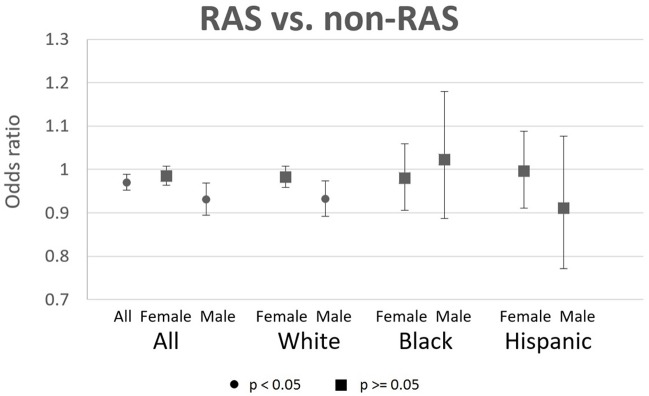
Fig 2. Odds ratios of AD incidence for RAS users relative to non-RAS users, with 95% CIs.
Each OR is the result from a separate logistic regression, which compares RAS AHT users to non-RAS AHT users in the same sex-race/ethnicity subgroup. Sample of 2009–2013 Medicare enrollees with use of antihypertensive (AHT) prescription drugs (angiotensin converting enzyme inhibitors (ACEIs), angiotensin-II receptor blockers (ARBs), beta-blockers, calcium channel blockers, loop diuretics, and thiazide diuretics). Sample sizes of person-years are: 4215338 for all, 2820575 for females, 1394763 for males, 2324640 for white females, 1177028 for white males, 221664 for black females, 73857 for black males, 154142 for Hispanic females, and 76745 for Hispanic males. Users defined as those with 90 possession days and 2 claims in year t-1 and year t-2. Sample restricted to person-years with 3 years fee-for-service, 3 years Part D, age 67+, no deaths in the reference year (year t), no prior AD diagnoses, and no prior use of acetylcholinesterase inhibitors (AChEIs) or memantine. RAS (renin angiotensin system) acting drugs are ACEIs and ARBs. Controls are age, age squared, sex, race, education, income quartiles, statin use (t-1), years since hypertension diagnosis, HCC comorbidity index, number of physician visits, and indicators for past diagnoses of diabetes, atrial fibrillation, acute myocardial infarction, stroke, and hyperlipidemia. Standard errors are clustered at the county level.