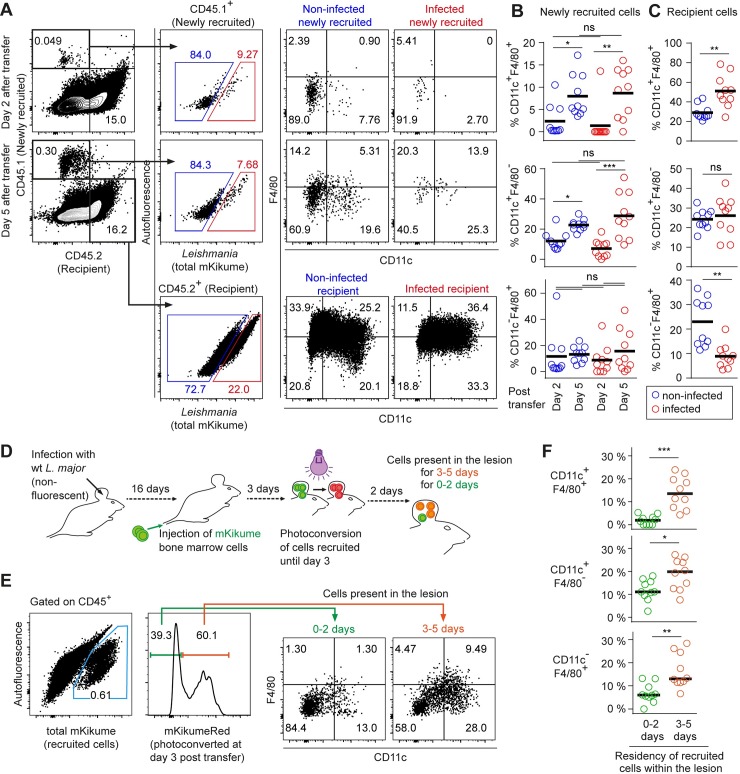
Fig 8. L. major infects newly recruited monocyte-derived cells independently of their differentiation stage.
(A) Flow cytometry analysis of C57BL/6 (CD45.2) mice infected with LmSWITCH, adoptively transferred 2 or 5 days before analysis with CD45.1 bone marrow cells. Gating strategy on CD45.1+ (newly recruited) and CD45.2+ (recipient) infected and non-infected cells. (B) Quantification of infected (red) and non-infected (blue) CD11c+ F4/80+ double positive (upper panel) and CD11c+ F4/80- single positive (lower panel) among newly recruited cells at day 2 and day 5 post adoptive transfer. (C) Analysis as in (B) for recipient cells. (D) Experimental strategy to identify newly recruited cells present for 0–2 versus 3–5 days post adoptive transfer. Mice infected with non-fluorescent L. major wild type received mKikume-expressing bone marrow cells 5 days prior to analysis. Photoconversion at 3 days post transfer identifies cells recruited to the site of infection until day 3 in mKikume red fluorescence, while cells recruited after photoconversion (0–2 days prior to analysis) exhibit green mKikume fluorescence. (E) After gating on CD45+ cells, all adoptively transferred cells are identified by total mKikume fluorescence. Transferred cells which were at the infection site since 0–2 days (showing no red fluorescence) can be clearly distinguished from cells that were recruited to the site of infection 3–5 days before analysis (and thus photoconverted, showing a high red fluorescence). The two populations were analyzed for CD11c and F4/80 expression. (F) Quantitative analysis of CD11c+ F4/80+ double positive (upper panel) and CD11c+ F4/80- single positive (lower panel) present at the site of infection for 0–2 days (green) and 3–5 days (red), respectively. Each dot represents one mouse ear. Horizontal bars denote the mean. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant.