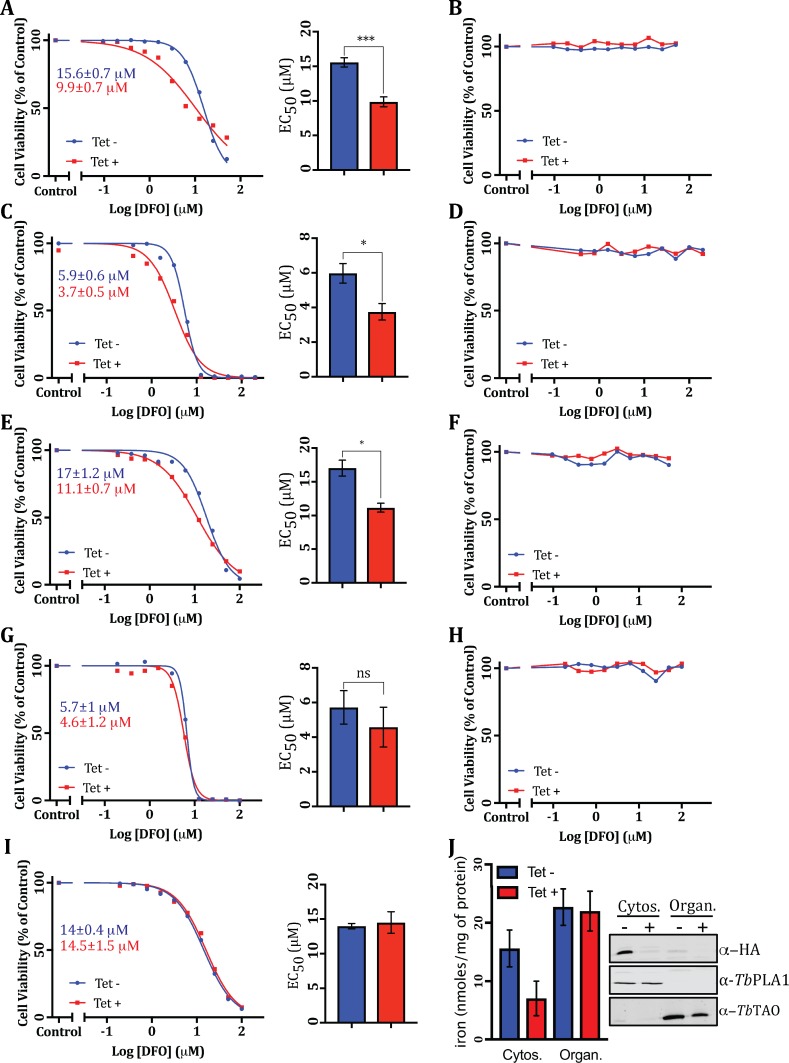
Fig 3. Knockdown of CIA members affects iron levels and sensitivity to iron depletion.
Wild type (WT), TbCIA2B, and TbMMS19 RNAi cells were grown without (blue, Tet -) or with (red, Tet +) tetracycline for 24 hours and then treated with different concentrations of deferoxamine (DFO). After 2 or 3 days of incubation (PCF and BSF parasites, respectively), cell growth was measured by the Resazurin method for determination of EC50s. Representative DFO concentration-response curves are shown in (A) TbCIA2B PCF, (C) TbCIA2B BSF, (E) TbMMS19 PCF, (G) TbMMS19 BSF, or (I) WT PCF. Representative plots of DFO pre-incubated with an excess of iron before adding to (B) TbCIA2B PCF, (D) TbCIA2B BSF, (F) TbMMS19 PCF, (H) TbMMS19 BSF. The values shown in the inset of the curves are the mean DFO EC50s for induced or uninduced cultures. The bar charts on the right side of the curves are the mean ± SEM EC50s of 3 independent experiments performed in quadruplicate. ns = non-significant; * = p< 0.05; *** = p<0.001 (two tailed paired t test). (J) PCFTbCIA2B RNAi cells were grown for 4 days in the presence (red) or absence (blue) of tetracycline and the content of iron bound to proteins was measured in the cytosolic and organellar fractions of digitonin permeabilised parasites. The purity of the cellular fractions was validated by Western blot using anti-HA (TbCIA2B-HA), anti-TbPLA1 (cytosolic marker), or anti-TbTAO (mitochondrial marker).