Abstract
Background: The Palliative Performance Scale (PPS) has been widely used for survival prediction among patients with cancer; however, few studies have reviewed PPS scores in heterogeneous palliative care populations across multiple care settings.
Objective: The aim of this systematic review was to determine how the PPS tool has been used to estimate survival at the end of life.
Methods: This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. PubMed, Embase, and the Cochrane Library were searched for the existing literature published from 2008 to 2017. We synthesized study characteristics, the PPS scores at baseline, and primary outcomes, and explored differences in survival estimates by diagnosis. The quality of the studies was assessed using the Good ReseArch for Comparative Effectiveness (GRACE) checklist.
Results: Seventeen studies were included in this review (nine with cancer and eight with mixed diagnoses). All included studies reported that the PPS exhibited a significant association with survival. Survival estimates ranged from 1 to 3 days for patients with PPS scores of 10% compared with 5 to 36 days for those with scores of 30%. The categorical cut-points for the PPS scores were not consistently reported across studies.
Conclusion: This review provides a broad overview on the prognostic value of the PPS tool for survival among multiple patient populations across care settings. Consistent reporting of PPS scores would facilitate the comparison of survival estimates across end-of-life diagnoses.
Keywords: : hospices, palliative care, Palliative Performance Scale, prognosis, review, survival
Introduction
Knowing how much time is left is a critical issue for terminally ill palliative care patients. Accurate survival prognostication enables patients and their families to make better decisions about goals of care and to prepare for the end of life.1,2 It is also important for healthcare providers to proactively refer patients for hospice care at the appropriate time.1 A clear prognosis helps hospice organizations better allocate the intensity of palliative care services, including end-of-life treatments.3 Given the importance of accurate survival estimates, valid measures are needed.
The Palliative Performance Scale (PPS)4 is a reliable and valid tool that has been used to measure functional performance of,2,7 and to predict survival among, palliative care cancer patients.6,10–13 The PPS is a modification of the Karnofsky Performance Scale,8 which was originally developed for cancer patients and later adapted to be more generalizable to other end-of-life diagnosis. The PPS scores measure five functional domains: ambulation, activity level and evidence of disease, self-care, oral intake, and level of consciousness (Appendix A). Each of the five domains is divided into 11 levels ranging from 0% to 100% in 10%-point intervals, with 0% indicating death and 100% being fully ambulatory and healthy. The PPS is simple to use in various healthcare settings,1,9 and the assessment of the PPS can be conducted by multiple healthcare professionals, including physicians and registered nurses.1
This systematic literature review critically evaluates the relationship between PPS scores and length of survival in patients with end-of-life diagnoses. This is particularly relevant because palliative and hospice services have expanded over the last decade to include multiple end-of-life diagnoses after initially focusing on patients with cancer. The aim of this systematic literature review was to determine how the PPS tool has been used to estimate survival at the end of life in both patients with cancer and patients with other end-of-life diagnoses.
Methods
This systematic review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16
Search strategy and selection criteria
A search of the literature was conducted between September and October 2017 using the following electronic databases: PubMed, Embase, and the Cochrane Library. The search strategy was developed through a literature review and consultation with an informationist in the health sciences library at Columbia University. The searches used MeSH terms for PubMed, Emtree terms for Embase, and free text terms. Boolean searching techniques using “AND” and “OR” included the following search terms: “palliative performance scale,” “prognosis,” “mortality,” “survival,” “rehospitalization,” and “length of services.” More detailed search terms used for each database are outlined in Appendix B.
Studies were included if they met the following criteria: (1) published between January 2008 and October 2017, (2) written in English, (3) original research study with data, (4) studies that investigated survival times or survival/mortality proportions after the PPS scores were administered, and (5) studies that examined either the prognostic value of the PPS tool for survival or association between the PPS and survival/mortality. Studies were excluded if they (1) provided only a discussion, opinion, commentary, review, or editorial; (2) were a published conference abstract only or presentation slides; (3) examined healthcare providers' outcomes (e.g., level of knowledge, perspectives) without patient outcomes; or (4) involved patients who were <18 years old.
Data extraction and analysis
Titles were screened (D.B.), and abstracts were independently reviewed by two reviewers (D.B. and R.M.C.) to identify eligible studies. During the abstract review, the two reviewers met and compared opinions on included and excluded articles, and consensus was reached through discussion when there were different opinions. Full-text articles were reviewed by two reviewers (D.B. and R.M.C.). The following data were extracted in the final review and presented in a table of an Excel spreadsheet: (1) author, (2) year of publication, (3) country, (4) study design, (5) study setting, (6) patient characteristics (number of participants at baseline, mean age, gender, race/ethnicity, diagnosis type, and PPS scores at baseline), and (7) patient outcomes measured. After the coding process, the extracted data were analyzed and synthesized using descriptive statistics.
Quality assessment
The Good ReseArch for Comparative Effectiveness (GRACE) checklist17 was used to assess the quality of the included studies. This tool was chosen because it is validated and designed to systematically assess the rigor of observational research studies.17,18 The GRACE checklist consists of 11 items, including 6 related to data and 5 related to methods. The 11 items are scored by dichotomized responses of sufficient (“+”) or insufficient (“–”). Four reviewers (D.B., D.R., L.J., and R.M.C.) independently assessed the quality of the studies. Consensus was reached through discussion when there were discrepancies.
Results
Study selection
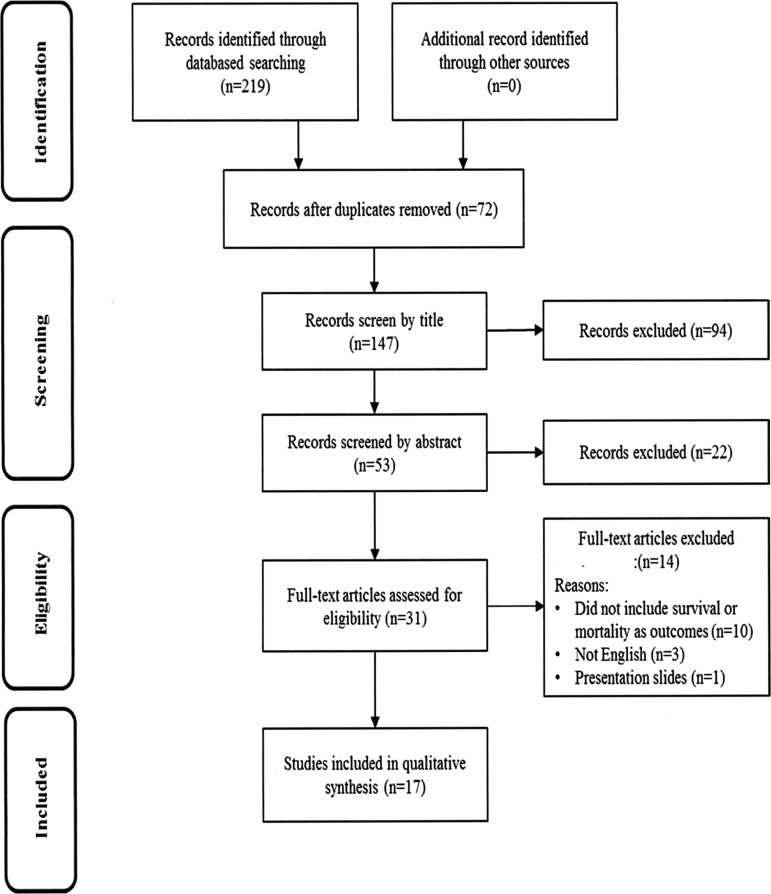
A flow diagram illustrating our review process is shown in Figure 1. The initial search yielded 219 articles from three electronic databases. Of the 219 articles, 72 articles were duplicates and removed. Titles of the remaining 147 articles were screened based on the inclusion/exclusion criteria of this review for eligibility. The titles for 53 of these articles passed an initial screening, and the abstracts were more closely reviewed. During the abstract review, 22 articles were excluded because they did not meet the inclusion criteria of this review. The remaining 31 articles were included for the full-text review, and then 14 articles were excluded for several reasons (e.g., not in English, presentation slides). In total, 17 articles were included for the final review.
FIG. 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study information
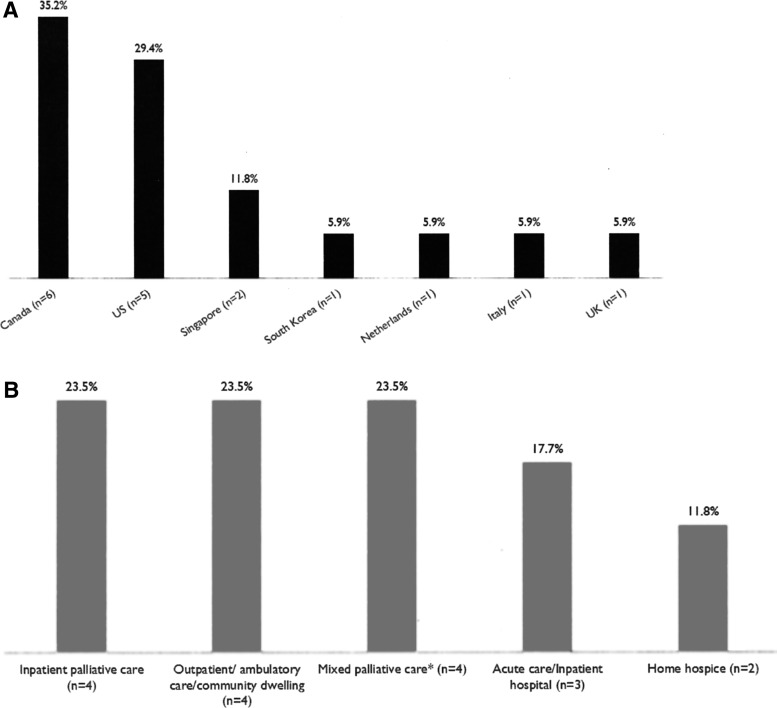
All 17 reviewed studies were published between 2009 and 2017 in seven countries, with the majority in Canada (n = 6; 35%) and the United States (n = 5; 29%) (Fig. 2A). The most common study design was a prospective study design (n = 9; 53%), followed by retrospective (n = 8; 47%). Studies included in this review were conducted in inpatient palliative care units (n = 4; 23.5%), outpatient/ambulatory care/community-dwelling settings (n = 4; 23.5%), and mixed palliative care settings (n = 4; 23.5%), including skilled nursing facilities, inpatient palliative care units, home hospice settings, and outpatient palliative care settings (Fig. 2B).
FIG. 2.
Characteristics of included studies. (A) Countries. (B) Study settings. *Mixed palliative care settings included skilled nursing facilities, inpatient palliative care units, home hospice settings, and outpatient palliative care settings.
Overall, nine studies (53%) included patients diagnosed with cancer, and eight studies (47%) included palliative care patients with mixed diagnoses such as cancer, cardiovascular disease, and stroke. The majority of the studies (n = 11; 65%) recorded patients' PPS scores by clinicians (physician or registered nurse) who cared for patients in the study settings. Of the remaining six studies, four did not clearly report who measured and recorded the PPS scores, while two studies stated that patients' PPS scores were recorded by research team members (e.g., physician, registered nurse) who did not involve in caring for patients in the study settings (Tables 1 and 2).
Table 1.
Summary of Palliative Performance Scale Survival among Patients with Cancer (n = 9)
| Author, county, year | n | Study settings, years of data collection | Age, gender | Diagnoses | PPS recorded by | PPS at baseline (n, %) | Primary outcomes |
|---|---|---|---|---|---|---|---|
| Jang, Canada, 2014 | 1655 | Oncology Palliative Care Clinic at Princess Margaret Cancer Centre 2007–2010 |
Median: 65 Male: 809 (49%) Female: 846 (51%) |
GI, lung, genitourinary, breast, gynecological, head and neck, CNS, hematological, other | Physician | PPS 10–30%: 24 (1%) PPS 40–50%: 315 (19%) PPS 60–70%: 784 (48%) PPS 80–100%: 508 (31%) |
Survival time (median days, 95% CI) PPS 10–30%: 22 (12–102) PPS 40–50%: 51 (44–60) PPS 60–70%: 115 (105–131) PPS 80–100%: 221 (197–244) |
| Jansen, Netherland, 2015 | 78 | Residential hospice 2010 |
Mean: 70.6 Male: 47 (60%) Female: 31 (40%) |
Terminal primary cancer diagnosis patients | Physician | PPS 10%: 1 (1%) PPS 20%: 14 (18%) PPS 30%: 8 (10%) PPS 40%: 21 (27%) PPS 50%: 21 (27%) PPS 60%: 10 (13%) PPS 70%: 3 (4%) |
Survival time (median days, IQR) PPS 10–20%: 6 (4–11) PPS 30–50%: 17.5 (11.75–41.5) PPS >50%: 41 (22.5–75) |
| Kim, South Korea, 2014 | 415 | Kyungpook National University Hospital and Medical Center 2009–2011 |
Mean: 60.7 Male: 240 (57.8%) Female: 175 (42.2%) |
Terminal cancer patients (stomach, lung, colon, biliary track, pancreatic, liver, uterine cervical, breast, ovarian, prostate) | Not reported | Not recorded | Survival time PPS <30%: 3–4 weeks PPS cutoff point for survival <3 and <4 weeks was 30 or below AUC 3 weeks: 0.729 (95% CI 0.684–0.772) AUC 4 weeks: 0.771 (95% CI 0.727–0.810) |
| Maltoni, Italy, 2012 | 549 | Three Italian hospices 2009–2010 |
Median: 71 Male: 269 (49%) Female: 280 (51%) |
Gastrointestinal, respiratory, genitourinary, other | Physician | PPS 10–20%: 227 (41.3%) PPS 30–50%: 314 (57.2%) PPS ≥60%: 8 (1.5%) |
Survival time (median days, 95% CI) PPS 10–20%: 11 (9–13) PPS 30–50%: 32 (28–41) PPS ≥60%: 139 (67, n.r.) |
| Mei, Singapore, 2013 | 296 | Palliative Care Unit of Tan Tock Seng Hospital 2009–2011 |
Mean: 68.9 Male: 159 (53.72%) Female: 137 (46.28%) |
Breast, CUP, GI, GU, head and neck, MSK pulmonary, skin |
Not reported | PPSv2 10–30%: 140 (39.32%) PPSv2 40–60%: 199 (55.90%) PPSv2 70–100%: 17 (4.78%) |
Survival time (median days) PPS 10–30%: 19 PPS 40–60%: 43 PPS 70–100%: 59 |
| Myers, Canada, 2015 | 368 | Outpatient Palliative Care Clinic within the Odette Cancer Centre at Sunnybrook Health Sciences Centre 2011–2013 |
Mean: 64.8 Male: 160 (44%) Female: 208 (56%) |
Gastrointestinal, breast, lung, gynecological, genitourinary, head and neck; melanoma, hematology, unknown prim, other | Physician | PPS ≤40%: 18 (5%) PPS 50%: 68 (18%) PPS 60%: 113 (31%) PPS 70%: 99 (27%) PPS 80%: 59 (16%) PPS 90%: 11 (3%) Median PPS: 60 (range 20–90) |
Survival time (median days) PPS 40%: 28 PPS 50%: 71 PPS 60%: 104 PPS 70%: 173 PPS 80%: 197 PPS 90%: 226 |
| O'Mahony, United States, 2016 | 62 | Outpatient oncology clinic at University Medical Center (dates not recorded) | Mean: 63.7 Gender: unspecified |
Lung | Physician | PPS 40%: 1 (1.61%) PPS 50%: 10 (16.31%) PPS 60%:11 (17.74%) PPS 70%: 31 (50%) PPS 80%: 5 (8.06%) PPS 90%: 4 (6.45%) |
Survival time Changes in PPS scores between baseline and 1 month were not significantly associated with shorter survival time PPS was associated with survival time when stratified by median score at baseline |
| Seow, Canada, 2013 | 11,342 | Ambulatory setting 2007–2009 |
Mean: 65 Male: 4144 (52.58%) Female: 3738 (47.42%) |
Breast, CNS, gastrointestinal, genitourinary, gynecologic, hematology, head and neck, other, primary unknown sarcoma, skin, lung | Physician or nurse | PPS ≤20%: 40 (0.3%) PPS 30%: 109 (1%) PPS 40%: 263 (2.3%) PPS 50%: 800 (7.1%) PPS 60%: 1222 (10.8%) PPS 70%: 1794 (15.8%) PPS 80%: 2105 (18.6%) PPS 90%: 2431 (21.4%) PPS 100%: 2578 (22.7%) |
Survival time (median days) PPS 40%: 154 PPS 50%: 231 PPS 60%: 315 PPS 70%: 441 PPS 80%: 686 PPS 90%: >800 |
| Weng, United States, 2009 | 492 | Home hospice program 2005–2006 |
Mean: 67 Male: 219 (44.5%); Female: 273 (55.5%) |
Lung, GI, GU, breast, hematological, neurological head and neck, muscle, other | Nurse | PPS 10%: 32 (6.5%) PPS 20%: 35 (7.1%) PPS 30%: 57 (11.6%) PPS 40%: 181 (36.8%) PPS 50%: 122 (24.8%) PPS 60%: 44 (8.9%) PPS 70%: 18 (3.7%) PPS 80%: 2 (0.4%) PPS 90%: 0 (0%) PPS 100%: 1 (0.2%) |
Survival time (median days, SD) PPS 10%: 3 (6.2) PPS 20%: 5 (33.5) PPS 30%: 10 (19.6) PPS 40%: 19 (54.4) PPS 50%: 29.5 (59.6) PPS 60%: 35 (50.8) PPS 70%: 48 (60.7) PPS 80%: 17.5 (4.9) |
AUC, area under the curve; PBS, Palliative Performance Scale.
Table 2.
Summary of Palliative Performance Scale Survival among Patients with Mixed Diagnoses (n = 8)
| Author, county, year | n | Study settings, years of data collection | Age, gender | Diagnoses | PPS recorded by | PPS at baseline, (n, %) | Primary outcomes |
|---|---|---|---|---|---|---|---|
| Babcock, United States, 2016 | 123 | Emergency department in John Dempsey Hospital 2013 |
Mean: 78.7 Male: 48 (39%) Female: 75 (61%) |
Cardiac disease: 33 (27%) Infectious disease: 25 (21%) Abdominal pain: 20 (16%) Fracture: 6 (5%) Dehydration: 6 (5%) Cancer: 5 (4%) COPD: 3 (2%) Anemia: 3 (2%) Renal failure: 3 (2%) Miscellaneous: 19 (15%) |
Research team member (physician) | PPS mean: 70.7% PPS range: 30–100% |
Three-month survival proportions PPS 0–30%: 42.9% PPS 40–60%: 85.1% PPS 70–100%: 97.1% Six-month survival proportions (mean days) PPS 0–30%: 14.3% (107) PPS 40–60%: 74.5% (151) PPS 70–100%: 95.7% (175) |
| Chan, Singapore, 2013 | 400 | Tan Tock Seng Hospital (acute care hospital) 2009–2010 |
<45:10 (2.5%) 45–64: 99 (24.8) 65–74: 104 (26%) 75–84: 126 (31.5%) ≥85: 61 (15.3%) Male: 176 (44%) Female: 224 (56%) |
Cancer: 340 (85%) Noncancer: 60 (15%) |
Research team member (registered nurse) | PPSv2 10%: 15 (3.8%) PPSv2 20%: 9 (2.3%) PPSv2 30%: 104 (26%) PPSv2 40%: 104 (26%) PPSv2 50%: 87 (21.8%) PPSv2 60%: 44 (11%) PPSv2 ≥70%: 37 (9.3%) |
Survival time (median days, 95% CI) PPSv2 10%: 2 (1.33, 2.67) PPSv2 20%: 8 (0.0, 16.77) PPSv2 30%: 23 (10.51, 35.49) PPSv2 40%: 25 (17.89, 32.14) PPSv2 50%: 43 (28.04, 57.96) PPSv2 60%: 41 (23.36, 58.64) PPSv2 ≥70%: 116 (45.49, 186.51) |
| Downing, Canada, 2010 | 3328 | Victoria Hospice Society Palliative care unit 1993–2005 |
Mean: 70.2 Male: 1595 (48.1%) Female: 1723 (51.9%) |
Cancer: 2939 (88.3%) Noncancer: 389 (11.7%) |
Physician or nurse | PPSv2 10%: 221 (6.6%) PPSv2 20%: 335 (10.1%) PPSv2 30%: 1028 (30.9%) PPSv2 40%: 1098 (33%) PPSv2 50%: 508 (15.3%) PPSv2 60%: 128 (3.9%) PPSv2 70%: 10 (0.3%) |
Survival time (median days)a PPS 30%: ∼17 PPS 40%: ∼30 PPS 50%: ∼50 PPS 60%: ∼65 |
| Harris, United States, 2014 | 118,532 | Home, nursing home or hospice, hospital/inpatient unit in New Mexico, California,Florida, Pennsylvania, Wisconsin, Michigan, Ohio, Texas,and Kansas/Missouri 2008–2012 |
≥65: 97,068 (81.9%) <65: 21,464 (18.1%) Male: 53,944 (45.5%) Female: 64,588 (54.5%) |
Cancer: 45,601 (35.8%) Debility: 13,466 (11.4%) Cardiovascular disease: 16,522 (13.9%) Dementia: 11,931 (10.1%) Pulmonary: 8037 (6.8%) Stroke: 5559 (4.7%) Other: 17,416 (14.7%) |
Not reported | PPS <30%: 26,005 (21.9%) PPS 30%: 22,227 (18.8%) PPS 40%: 24,792 (20.9%) PPS 50%: 19,900 (16.8%) PPS ≥60%: 5731 (4.8%) Missing: 19,877 (16.8%) |
Six-month mortality Six-month mortality probability ranged from 32.6% to 99.5%. PPS scores, OR (95% CI) <30: reference 30: 0.22 40: 0.10 50: 0.07 >60: 0.04 |
| Lau, Canada, 2009 | 6066 | Victoria Hospice palliative care, home or palliative inpatient unit 1993–2005 |
Mean: 71.5 Male: 2892 (47.7%) Female: 3156 (52.0%) Unknown: 18 (0.3%) |
Cancer: 5097 (84.0%) Noncancer: 756 (12.5%) AIDS: 75 (1.2%); Cardiovascular: 328 (5.4%) Neurological: 69 (1.1%) Respiratory: 157 (2.6%) Others: 127 (2.1%) Not recorded: 213 (3.5%) |
Physician or nurse | Not recorded | Survival time (median days, 95% CI) PPS 10%: 1 (1–1) PPS 20%: 2 (2–2) PPS 30%: 5 (5–5) PPS 40%: 13 (12–14) PPS 50%: 28 (25–31) PPS 60%: 43 (38–48) PPS 70%: 63 (48–78) |
| Lau, Canada, 2009 | 513 | William Osler Health Centre in Toronto, Canada (inpatient and outpatient units) 2005–2006 |
Mean: 74.91 Male: 256 (49.9%) Female: 257 (50.1%) |
Cancer: 347 (67.6%) Noncancer: 166 (32.4%) |
Physician or nurse | PPSv2 10%: 27 (5.3%) PPSv2 20%: 71 (13.8%) PPSv2 30%: 118 (23%) PPSv2 40%: 56 (10.9%) PPSv2 50%: 88 (17.2%) PPSv2 60%: 56 (10.9%) PPSv2 70%: 81 (15.8) PPSv2 80%: 16 (3.1%) |
Survival time (median, days, 95% CI) PPSv2 10%: 2 (1–3) PPSv2 20%: 6 (4–8) PPSv2 30%: 12 (9–15) PPSv2 40%: 31 (15–47) PPSv2 50%: 35 (29–41) PPSv2 60%: 50 (33–67) PPSv2 70%: 110 (77–143) PPSv2 80%: 71 (0–196) |
| Linklater, United Kingdom, 2012 | 666 | Specialist palliative care unit, Primary, Secondary, nursing home (dates not recorded) | Unspecified Male: 281 (42.2%) Female: 385 (57.8%) |
Malignant: 397 (59.6%) Nonmalignant: 163 (24.5%) Dementia/frailty: 106 (15.9%) |
Physician or nurse | Not recorded | Survival time (median days, IQR) days PPS 10%: 3 (1–11) PPS 30%: 35.5 (17.25–123.5) PPS 50%: 52 (18–141) PPS 100%: 138 (115.5–335.5) |
| McGreevy, United States, 2017 | 153 | Surgical ICU 2012 |
Mean: 70.8 Male: 93 (60.8%) Female: 60 (39.2%) |
Traumatic brain injury | Not reported | PPS 50%: 6 (3.9%) PPS 60%: 9 (5.9%) PPS 70%: 20 (13.1%) PPS 80%: 23 (15%) PPS 90%: 41 (26.8%) PPS 100%: 54 (35.3%) |
In-hospital mortality In-hospital mortality: 28 (18.3%) In a model adjusting for age, injury severity score, and traumatic brain injury, PPS was a significant predictor of in-hospital mortality |
Estimated survival time in days based on Kaplan–Meier survival curves in the text.
PPS scores at baseline
Of the 17 studies, 13 (76%) reported the detailed PPS scores on admission or during a baseline interview. Of the four remaining studies, most either did not specify the initial PPS scores or reported only the PPS range, mean, or median. Among the 13 studies that reported the details of initial PPS scores, seven1,10,14,19–22 reported the initial PPS as a categorical variable that ranged from three to nine cut-points. Each of these seven studies chose different categorical cut-points. For example, Maltoni et al.20 reported three categories (PPS 10–20%, PPS 30–50%, PPS ≥60%). Of the 13 studies, the 6 remaining2,5,12,13,23,24 reported the initial PPS scores as discrete variables, which had different ranges of PPS scores (e.g., PPS 10–70%, PPS 40–90%). Of the six studies, two studies2,5 reported PPS ranging from 10% to 70%, while the four remaining studies reported various ranges. For example, O'Mahony et al.23 reported PPS scores ranging from 40% to 90%, while Lau et al.11 reported PPS scores ranging from 10% to 80%.
Patient outcomes measured
The included studies measured patient outcomes as either survival times (n = 14; 82%) or survival/mortality proportions (n = 3; 18%). PPS scores were represented as categorical or discrete variables within different cohorts. In this review, survival time was defined as the period between the date of admission or a baseline interview and the date of death. Thirteen studies (76%) included covariates in their prediction models when using the PPS to estimate survival times. The most common covariate was age (n = 10), followed by gender (n = 9) and diagnosis or cancer site (n = 8).
Survival estimates by PPS scores
As described above, most studies (n = 15; 88%) reported survival time or proportions predicted by PPS scores. Specifically, survival estimates among five studies with PPS scores of 10% ranged from one to three days.1,11–13,25 The survival ranges for patients who scored 30% on the initial PPS were substantially wider (5–36 days). To examine the accuracy of PPS scores in predicting survival, three studies (18%)10,20,26 measured the predictive validity of the PPS by using sensitivity, specificity, or the area under the curve (AUC). Mei et al.10 showed that the study model increased predictive accuracy of survival in patients within 90 days, with a positive predictive value (PPV) of 79% and an AUC of 0.70. Kim et al.26 reported that when the PPS scores are <30%, the predictive accuracy of the PPS for three- and four-week survival was acceptable (AUC = 0.729 and 0.771, respectively). The findings from these two studies differed from Maltoni et al.20 who found that the predictive accuracy of the PPS at 30 days was less than a PPV of 50%.
To better understand the use of the PPS for survival prediction based on patient diagnoses, we analyzed the prognostic value of the PPS tool on survival by dividing all included studies into two diagnosis groups (cancer vs. mixed diagnosis).
Survival estimates for cancer patients
The PPS survival among studies that only included cancer patients (n = 9) is summarized in Table 1. The studies were most commonly conducted in Canada (n = 3) and the United States (n = 2). The most common study settings were outpatient/ambulatory care/community-dwelling settings (n = 4), followed by inpatient palliative care units (n = 3). The number of participants at baseline ranged from 62 to 11,342. Of the nine studies, seven reported mean age of patients with cancer, which ranged from 61 to 71 years. Eight studies reported the gender of patients; four studies included a higher percentage of females, and the remaining four studies consisted of a higher percentage of males. Most studies (n = 8) did not examine the race or ethnicity of study participants; however, one study13 conducted in the United States reported that the largest racial or ethnic group was African American (46.3%), followed by Caucasian (30.9%). All but one of the studies2 reported specific types of participants' cancer diagnoses. Of the nine studies that included cancer patients, eight reported initial PPS scores, and each study chose to report different ranges of the PPS scores. Five of these studies reported categorical scoring of the PPS.10,14,19–21 However, these scoring categories differed considerably across studies, ranging from three to nine categories. Three studies2,13,23 reported the initial PPS scores as a discrete variable and had different ranges of PPS scores in their patient populations.
All nine studies reported detailed survival time stratified by initial PPS scores recorded on admission or during a baseline interview, and the survival time was reported by categorical or discrete variables of the PPS scores within different cohorts. All nine studies reported a significant association between PPS scores and survival among palliative care patients with advanced cancer.
Survival estimates for mixed diagnosis patients
The characteristics among eight of the studies with a mixed diagnosis palliative care sample are summarized in Table 2. The included studies were conducted in Canada (n = 3), the United States (n = 3), Singapore (n = 1), and the United Kingdom (n = 1). The most common study settings were mixed palliative care settings (n = 4), followed by acute care units/inpatient hospitals (n = 3). The number of participants in these studies ranged from 123 to 118,532. Of the eight studies, five5,9,11,12,24 reported the mean age of study participants ranging from 70 to 79 years. Seven1,5,9,11,12,22,25 of the eight studies included a higher percentage of females. Three studies9,22,24 conducted in the United States examined the race or ethnicity of the study participants, and revealed that the majority of the study participants were Caucasian.
Of the eight studies, five examined detailed initial PPS scores, and reported them with different ranges of the scores across the studies. Two studies1,22 reported categorical initial PPS scores using five and seven categories, respectively. Three studies5,24,27 reported the initial PPS scores as a discrete variable, and each chose different ranges of PPS scores (e.g., PPS 10% ∼ PPS 70%, PPS 10% ∼ PPS 80%, and PPS 50% ∼ PPS 100%).
Regarding patient outcomes estimated by categorical or discrete variables of the PPS scores, most studies (n = 6, 75%) measured survival time or proportions, and the remaining two studies22,24 analyzed mortality rates. All eight studies revealed that the PPS was a significant predictor for survival time or mortality rate.
Discussion
In this systematic literature review, we critically evaluated the relationship between PPS scores and survival times or proportions by synthesizing research across diverse care settings and analyzing differences in the characteristics of studies between two diagnosis groups (cancer vs. mixed diagnosis). The PPS was a significant predictor of survival for patients with both cancer and other end-of-life diagnoses. As such, this review confirms previous study findings among patients with cancer7 and supports the use of the PPS tool for estimating the length of survival among patients with mixed end-of-life diagnoses such as heart disease, pulmonary disease, and dementia.
Most studies included in this review were conducted in Canada and in the United States. This was consistent with findings from a previous review,7 and is most likely explained by the fact that the PPS was developed at the Victoria Hospice Society in Canada.25 Specifically, because there were already established research teams in Canada, the evidence base establishing the use of PPS as a tool for estimating survival is larger compared with other countries.
Both prospective and retrospective designs were used in the studies included in this review. Prospective designs were defined as studies in which researchers recruited participants to assess the PPS at baseline and collected follow-up data about survival length or proportions. Retrospective designs were defined as studies in which the researchers used medical record data to define PPS scores and survival/mortality after the point at which the patient had received treatment. The retrospective studies may be limited by less accurate measurements of PPS scores compared with prospective studies because trained researchers could not interview palliative care patients.28
Across studies, there was wide variation in how the PPS scores and subsequent survival were reported. Some studies examined the PPS scores using categorical variables, while others used discrete measures of the PPS. Specifically, studies reporting the categorical PPS scores included various numbers of categories and cut-points. Studies reporting the discrete measure of the PPS also described different ranges of the PPS and subsequent survival estimates. Moreover, these studies did not present the rationale regarding how or why they chose ways to categorize PPS scores or decided to use certain ranges of PPS scores. Downing et al.15 also reported the need for the consistency in reporting survival probabilities and length of survival based on the PPS scores. The lack of consistency in categorization of PPS scores hampers our ability to draw comparisons between studies utilizing survival analysis among different palliative care populations. Downing et al.15 suggested the following recommendations for consistently reporting survival based on the PPS scores: (1) reporting median survival times according to PPS levels; (2) using PPS quartiles with confidence intervals to convey information about early, mid, and late survivors; or (3) establishing a life table from PPS survival curves.
A majority of the studies focused on examining the association between the PPS and survival, while only three studies investigated the predictive accuracy of the PPS by using sensitivity, specificity, or AUC. Examining the optimal cutoff points of the PPS for the prediction of survival is essential to provide helpful information about when and which diagnoses the PPS tool is effectively predictive of survival. More studies investigating the predictive accuracy of the PPS in estimating survival across heterogeneous palliative care populations are needed to help healthcare providers predict accurate survival times using the PPS tool.
Limitations
This review included only studies written in English; thus, it may have missed studies that report the relationship between the PPS and survival in non-English-speaking countries. The PPS scores were not reported consistently across studies; thus, the heterogeneity in how PPS scores were reported in relation to survival times/proportions limited our ability to conduct a meta-analysis. Inconsistencies may also be introduced in survival estimation through the recent reorientation of palliative care away from specialized clinicians trained in palliative medicine toward a “palliative approach” in which palliative knowledge and expertise is integrated into models of care that do not formally specialize in palliative care.29 This issue may be an important topic for future reviews investigating the association of PPS scores with survival among patients nearing the end of life.
Conclusion
The findings from this review confirm that the PPS tool has been used more frequently to estimate survival in patients with cancer, and that there is a lot of opportunity to apply the tool to palliative care patients with different end-of-life diagnoses. In studies that include the PPS as a measure, there should be more consistency in how it is reported to support generalizability of the measure across study settings and disease diagnoses. Additional research is needed to determine evidence-based cut-points for different patient populations. Moreover, further research is needed in countries where the PPS is less widely used, such as countries in South America, Africa, Europe, and Asia, to obtain more reliable and validated measures for survival prediction by considering different races, ethnicities, and cultures. Specifically, Europe mainly uses the Karnofsky Performance Scale, and Japan incorporates the PPS into the Palliative Prognostic Index.30 More research is also needed on how the PPS tool can be used in other underrepresented palliative care patient populations such as those with heart disease. Finally, we recommend that the PPS is used in acute care or home care settings to identify those who should receive palliative care services earlier.
Appendix A.
Palliative Performance Scale
| PPS level (%) | Ambulation | Activity and evidence of disease | Self-care | Intake | Conscious level |
|---|---|---|---|---|---|
| 100 | Full | Normal activity & work No evidence of disease | Full | Normal | Full |
| 90 | Full | Normal activity & work No evidence of disease | Full | Normal | Full |
| 80 | Full | Normal activity & work No evidence of disease | Full | Normal or Reduced | Full |
| 70 | Reduced | Unable Normal Job/Work Significant disease | Full | Normal or Reduced | Full |
| 60 | Reduced | Unable hobby/house work Significant disease | Occasional assistance necessary | Normal or Reduced | Full or Confusion |
| 50 | Mainly Sit/Lie | Unable to do any work Extensive disease | Considerable assistance required | Normal or Reduced | Full or Confusion |
| 40 | Mainly in Bed | Unable to do most activity Extensive disease | Mainly assistance | Minimal to sips | Full or Drowsy +/− Confusion |
| 30 | Totally Bed Bound | Unable to do any activity Extensive disease | Total Care | Mouth care only | Full or Drowsy +/− Confusion |
| 20 | Totally Bed Bound | Unable to do any activity Extensive disease | Total Care | Full or Drowsy +/− Confusion | |
| 10 | Totally Bed Bound | Unable to do any activity Extensive disease | Total Care | Drowsy or Coma +/− Confusion | |
| 0 | Death | - | - | - | - |
PPS = Palliative Performance Scale.
Appendix B.
Search Terms
| PubMed | Embase | Cochrane library | |
|---|---|---|---|
| Phase 1. | “palliative performance scale”[tiab] OR ppsv2[tiab] | ‘palliative performance scale'/exp OR ‘palliative performance scale':ti,ab OR ppsv2:ti,ab OR ppsv2 | “palliative performance scale”:ti,ab OR ppsv2:ti,ab |
| Phase 2. | “prognosis”[mesh] OR prognosis[tiab] OR prognos*[tiab] OR predict*[tiab] OR “mortality”[mesh] OR mortality[tiab] OR “survival” [mesh] OR survival[tiab] OR rehospitalization[tiab] OR rehospitalization[tiab] OR length of service*[tiab] | ‘prognosis'/exp OR ‘prognosis':ti,ab OR ‘prognos*':ti,ab OR ‘predict*':ti,ab OR ‘mortality'/exp OR ‘mortality':ti,ab OR ‘survival'/exp OR ‘survival':ti,ab OR ‘hospital readmission'/exp OR ‘rehospitalization':ti,ab OR ‘re-hospialization':ti,ab | prognosis (mesh) OR prognos*:ti,ab OR predict*:ti,ab OR mortality (mesh) OR mortality:ti,ab OR survival (mesh) OR survival:ti,ab OR rehospitalization:ti,ab OR rehospitalization: ti,ab OR length of service*:ti,ab |
| Phase 1 AND Phase 2 | |||
Acknowledgments
This work was supported by the National Institute of Nursing Research of the National Institutes of Health for providing funding for D.B. under Award T32 NR007969. The authors gratefully acknowledge the Eugenie and Joseph Doyle Research Partnership Fund from the Visiting Nurse Services of New York for providing pilot funds for this study and the National Institute of Nursing Research of the National Institutes of Health for providing funding for R.M.C. under Award Number K99 NR016275.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chan EY, Wu HY, Chan YH: Revisiting the Palliative Performance Scale: Change in scores during disease trajectory predicts survival. Palliat Med 2013;27:367–374 [DOI] [PubMed] [Google Scholar]
- 2.Jansen WJJ, Buma S, Gootjes JRG, et al. : The palliative performance scale applied in high-care residential hospice: A retrospective study. J Palliat Med 2015;18:67–70 [DOI] [PubMed] [Google Scholar]
- 3.Chow R, Chiu N, Bruera E, et al. : Inter-rater reliability in performance status assessment among health care professionals: A systematic review. Ann Palliat Med 2016;5:83–92 [DOI] [PubMed] [Google Scholar]
- 4.Chewaskulyong B, Sapinun L, Downing M: Reliability and validity of the Thai translated version (Thai PPS adult suandok) of the palliative performance scale (PPSV2). J Thorac Oncol 2011;6:S583–S584 [DOI] [PubMed] [Google Scholar]
- 5.Downing GM, Lesperance M, Lau F, Yang J: Survival implications of sudden functional decline as a sentinel event using the palliative performance scale. J Palliat Med 2010;13:549–557 [DOI] [PubMed] [Google Scholar]
- 6.Al Saleh K, Al Sayed N, Abd-El-Gawad W, et al. : Palliative performance scale differences between patients referred by specialized cancer center and general hospitals to the palliative care center in Kuwait. J Clin Oncol 2015;33Se 20541 [Google Scholar]
- 7.Simmons CPL, McMillan DC, McWilliams K, et al. : Prognostic tools in patients with advanced cancer: A systematic review. J Pain Symptom Manage 2017;53:962–970.e910 [DOI] [PubMed] [Google Scholar]
- 8.Evans C, McCarthy M: Prognostic uncertainty in terminal care: Can the Karnofsky index help? Lancet (London, England) 1985;1:1204–1206 [DOI] [PubMed] [Google Scholar]
- 9.Babcock M, Gould Kuntz J, Kowalsky D, et al. : The Palliative Performance Scale predicts three- and six-month survival in older adult patients admitted to the hospital through the emergency department. J Palliat Med 2016;19:1087–1091 [DOI] [PubMed] [Google Scholar]
- 10.Mei AHY, Jin WLC, Hwang MKY, et al. : Value of the palliative performance scale in the prognostication of advanced cancer patients in a tertiary care setting. J Palliat Med 2013;16:887–893 [DOI] [PubMed] [Google Scholar]
- 11.Lau F, Downing M, Lesperance M, et al. : Using the Palliative Performance Scale to provide meaningful survival estimates. J Pain Symptom Manage 2009;38:134–144 [DOI] [PubMed] [Google Scholar]
- 12.Lau F, Maida V, Downing M, et al. : Use of the Palliative Performance Scale (PPS) for end-of-life prognostication in a palliative medicine consultation service. J Pain Symptom Manage 2009;37:965–972 [DOI] [PubMed] [Google Scholar]
- 13.Weng LC, Huang HL, Wilkie DJ, et al. : Predicting survival with the Palliative Performance Scale in a minority-serving hospice and palliative care program. J Pain Symptom Manage 2009;37:642–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seow H, Barbera L, Dudgeon D, et al. : The association of the palliative performance scale and hazard of death in an ambulatory cancer population. J Palliat Med 2013;16:156–162 [DOI] [PubMed] [Google Scholar]
- 15.Downing M, Lau F, Lesperance M, et al. : Meta-analysis of survival prediction with Palliative Performance Scale. J Palliat Care 2007;23:245–252; discussion 252–244 [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151:264–269, w264 [DOI] [PubMed] [Google Scholar]
- 17.Dreyer NA, Velentgas P, Westrich K, Dubois R: The GRACE checklist for rating the quality of observational studies of comparative effectiveness: A tale of hope and caution. J Manag Care Spec Pharm 2014;20:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyer NA, Bryant A, Velentgas P: The GRACE checklist: A validated assessment tool for high quality observational studies of comparative effectiveness. J Manag Care Spec Pharm 2016;22:1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang RW, Caraiscos VB, Swami N, et al. : Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract 2014;10:e335–e341 [DOI] [PubMed] [Google Scholar]
- 20.Maltoni M, Scarpi E, Pittureri C, et al. : Prospective comparison of prognostic scores in palliative care cancer populations. Oncologist 2012;17:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers J, Kim A, Flanagan J, Selby D: Palliative performance scale and survival among outpatients with advanced cancer. Support Care Cancer 2015;23:913–918 [DOI] [PubMed] [Google Scholar]
- 22.Harris PS, Stalam T, Ache KA, et al. : Can hospices predict which patients will die within six months? J Palliat Med 2014;17:894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Mahony S, Nathan S, Mohajer R, et al. : Survival prediction in ambulatory patients with stage III/IV non-small cell lung cancer using the Palliative Performance Scale, ECOG, and lung cancer symptom scale. Am J Hosp Palliat Care 2016;33:374–380 [DOI] [PubMed] [Google Scholar]
- 24.McGreevy CM, Bryczkowski S, Pentakota SR, et al. : Unmet palliative care needs in elderly trauma patients: Can the Palliative Performance Scale help close the gap? Am J Surg 2017;213:778–784 [DOI] [PubMed] [Google Scholar]
- 25.Linklater G, Lawton S, Fielding S, et al. : Introducing the Palliative Performance Scale to clinicians: The Grampian experience. BMJ Support Palliat Care 2012;2:121–126 [DOI] [PubMed] [Google Scholar]
- 26.Kim AS, Youn CH, Ko HJ, Kim HM: The survival time of terminal cancer patients: Prediction based on clinical parameters and simple prognostic scores. J Palliat Care 2014;30:24–31 [PubMed] [Google Scholar]
- 27.Lau F, Bell H, Dean M, et al. : Use of the Palliative Performance Scale in survival prediction for terminally ill patients in Western Newfoundland, Canada. J Palliat Care 2008;24:282–284 [PubMed] [Google Scholar]
- 28.Euser AM, Zoccali C, Jager KJ, Dekker FW: Cohort studies: Prospective versus retrospective. Nephron Clin Pract 2009;113:c214–c217 [DOI] [PubMed] [Google Scholar]
- 29.Sawatzky R, Porterfield P, Lee J, et al. : Conceptual foundations of a palliative approach: A knowledge synthesis. BMC Palliat Care 2016;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Kock I, Mirhosseini M, Lau F, et al. : Conversion of Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status (ECOG) to Palliative Performance Scale (PPS), and the interchangeability of PPS and KPS in prognostic tools. J Palliat Care 2013;29:163–169 [PubMed] [Google Scholar]