Abstract
Background:
Symptom clusters among adults with atrial fibrillation have previously been identified but no study has examined the relationship between symptom clusters and outcomes.
Aims:
The purpose of this study was to identify atrial fibrillation-specific symptom clusters, characterize individuals with each cluster, and determine whether symptom cluster membership is associated with healthcare utilization.
Methods:
This was a cross-sectional secondary data analysis of 1,501 adults from the Vanderbilt Atrial Fibrillation Registry with verified atrial fibrillation. Self-reported symptoms were measured with the University of Toronto Atrial Fibrillation Severity Scale. We used hierarchical cluster analysis (Ward’s method) to identify clusters and dendrograms, pseudo F, and pseudo T-squared to determine the ideal number of clusters. Next, we used regression analysis to examine the association between cluster membership and healthcare utilization.
Results:
Males predominated (67%) and the average age was 58.4 years. Two symptom clusters were identified, a Weary cluster (3.7%, n=56, fatigue at rest, shortness of breath at rest, chest pain, and dizziness) and Exertional cluster (32.7%, n=491, shortness of breath with activity and exercise intolerance). Several sociodemographic and clinical characteristics varied by symptom cluster group membership, including age, gender, AF type, BMI, comorbidity status, and treatment strategy. Women were more likely to experience either cluster (p < 0.001). The Weary cluster was associated with nearly triple the rate of emergency department utilization (IRR 2.8, p<0.001) and twice the rate of hospitalizations (IRR 1.9, p<0.001).
Conclusion:
We identified two symptom clusters. The Weary cluster was associated with a significantly increased rate of healthcare utilization.
Keywords: atrial fibrillation, symptom cluster
Introduction
More than 33 million individuals are estimated to have atrial fibrillation (AF) globally.1 Individuals with AF are typically older adults with cardiovascular comorbidities, such as hypertension, coronary artery disease, valve disorders, and heart failure.2 Further, AF increases the risk of ischemic stroke nearly 5-fold, heart failure approximately 3-fold, and is associated with increased mortality.3, 4 As a result of the high comorbidity burden, AF management is often complex and challenging, further complicating the care of older adults with multiple chronic conditions.
Treatment for AF is centered on integrated management to prevent complications and control symptoms via: 1) determining hemodynamic stability and symptom severity, 2) treating precipitating factors and underlying conditions, 3) preventing stroke, 4) heart rate control, and/or 5) rhythm control strategies.5 Symptom management is a primary AF treatment goal, and symptoms are a major predictor of hospitalizations among individuals with AF.6 AF symptoms differ drastically between individuals, with some people experiencing severe symptoms and other experiencing little to no symptoms. A thorough understanding of the divergent AF symptom experience is lacking, and little is known regarding the precise mechanisms causing AF symptoms.7 Evidence to date suggests that physiologic factors alone (e.g. left ventricular ejection fraction) do not fully explain AF symptom variability.7 Further, AF symptoms do not always correlate well with episodes of arrhythmia, making it challenging to know the best approach to symptom management for some individuals.8 Similarly, some individuals with heart failure perceive high levels of fluid overload even when objective data (intrathoracic impedance monitoring) indicates low congestion.9 Gaps in knowledge regarding AF symptom variability limits the ability of clinicians to develop personalized, precision approaches to AF symptom management.
The examination of symptom clusters is a new area of cardiovascular symptom research.10, 11 Symptom clusters are defined as groups of 2 or more symptoms that are related due to shared mechanisms, covariance, or effect on outcomes.12 Several authors have identified cardiovascular symptom clusters,10 with some authors discovering an association between specific clusters and health outcomes.13–15 If AF symptom clusters are identified that suggest a shared underlying mechanism or shared effect on outcomes, that information can be used to develop personalized approaches to AF symptom management. One prior study used cluster analysis to identify AF symptom clusters, characterized as vagal (nausea, diaphoresis), tired (fatigue, weakness, syncope/dizziness, dyspnea), and heart (palpitations, chest pain),11 but these clusters have yet to be replicated in subsequent studies. The purpose of this study was to: 1) identify AF-specific symptom clusters, 2) characterize the individuals with each, and 3) determine whether symptom cluster membership is associated with healthcare utilization.
Methods
We conducted a cross-sectional secondary data analysis using de-identified data from the Vanderbilt Atrial Fibrillation Registry (VAFR).16 VAFR is a single center clinical biorepository that prospectively enrolled adults with AF and their family members between 2002 and 2015. This investigation conforms with the principles outlined in the Declaration of Helsinki. This secondary data analysis was approved by the University of Pennsylvania IRB (protocol #824118).
Study Population
Consecutively enrolled patients from Vanderbilt cardiology clinics, emergency department, and inpatient services were captured in VAFR.16 Inclusion requirements for VAFR were documented AF or atrial flutter and age ≥ 18 years. AF was documented on an electrocardiogram (ECG), Holter monitor, rhythm strip, or event recorder. AF was defined as replacement of p-waves with rapid oscillations that varied in size, shape, and timing, were accompanied by irregular ventricular response when atrioventricular conduction was intact, and lasted a minimum of 30 seconds. Individuals were excluded from VAFR if AF was only present within the first 90 days after cardiac surgery or were unable/unwilling to provide informed consent.
Our sample consists of the 1,501 adults enrolled in the VAFR clinical registry between 2002 and 2015 with documented AF and a completed baseline symptom survey. We excluded from our analysis individuals with atrial flutter but not AF, and individuals who did not complete a baseline symptom survey.
Measurement of Variables
Demographic and clinical characteristics.
Upon enrollment in VAFR, a sociodemographic, medical, and drug history was obtained for all participants using an investigator designed form in RedCap17 to standardize data collection. A combination of patient-reported and medical record data was collected by study personnel (registered nurses), who were trained in the study protocol and use of a detailed study codebook. We used the following variables to characterize participants in our study: age at consent, gender, ethnicity, body mass index (BMI), left ventricular ejection fraction, left atrial diameter, AF type (paroxysmal, persistent, or permanent), age of AF onset, current use of anti-arrhythmic medication, current use of other cardioactive medications, history of one or more ablation, history of coronary bypass, heart failure, coronary disease, valve disease, hypertension, and CHADS2 score. The CHADS2 score is used to estimate stroke risk; scores range from 0 (least risk) to 6 (most risk) and are calculated by assigning one point each for the presence of heart failure (C), hypertension (H), age 75 or older (A), or diabetes (D), and two points for prior stroke/transient ischemic attack (S).18 Although a newer stroke risk score, the CHA2DS2-VASc,19 is currently recommended in clinical guidelines,5 the CHA2DS2-VASc was not developed at the time VAFR was initiated. We calculated history of AF by subtracting age of AF onset from age at consent. Paroxysmal AF was defined as AF lasting ≥ 30 seconds and terminating spontaneously. Persistent AF was defined as AF that lasting ≥ 7 days and requiring electrical/chemical cardioversion. Permanent AF was defined as continuous AF for which the decision was made not to restore sinus rhythm. Left atrial diameter and left ventricular ejection fraction were recorded on all participants from the echocardiogram or magnetic resonance imaging performed closest to time of enrollment.
Atrial fibrillation symptoms.
Participants completed the University of Toronto AF Severity Scale (AFSS) upon enrollment.16, 20 The AFSS is a 19-item survey composed of three sections: The first measures general life satisfaction and the global frequency, duration, and severity of AF episodes, the second measures healthcare utilization, and the third is a symptom subscale that measures the presence/frequency of seven of the most common AF symptoms (palpitations, shortness of breath at rest, shortness of breath with activity, exercise intolerance, dizziness, fatigue at rest, and chest pain).20 All measures used from the AFSS in this study were obtained from the participant at the same study timepoint (enrollment). Specifically, the following is asked of each specific symptom: how often have you been bothered by (palpitations) in the past 4 weeks. Subjects respond on a 6-point Likert scale ranging from none (0) to a great deal (5), and total scores for the symptom subscale range from 0 to 35. The internal consistency (Cronbach’s α) for AF burden is 0.94.21 Internal consistency and test-retest reliability for the symptom subscale have not been reported, however the AFSS has been used in the validation of other AF-specific disease severity and quality of life scales, including the Canadian Cardiovascular Society Severity in AF scale (CCS-SAF).22 We used the symptom subscale of the AFSS as our measure for the symptom cluster analysis.
Healthcare utilization.
The second section of the AFSS measures participants’ utilization of healthcare services. Specifically, participants report the number of AF-related hospitalizations, ED visits, specialist clinic visits, and cardioversions they had within the past 12-montths. We examined hospitalizations and ED visits because they represent healthcare utilization that could potentially be reduced with improved symptom management. The healthcare utilization section of the AFSS has a 3-month test-retest reliability of 0.71.21
Statistical Analysis
All analyses were conducted using SAS version 9.4 (Cary, North Carolina). Standard descriptive statistics were used to describe the data. Clusters were identified using cluster analysis, which refers to a set of graphical and statistical techniques that simultaneously maximize the heterogeneity between clusters and the homogeneity within clusters.23 Clusters were based on responses to the 7 symptom variables. Specifically, we used agglomerative hierarchical cluster analysis, using Ward’s method and Euclidean distance as our dissimilarity measure. Empirical studies indicate Ward’s method is the preferred approach, due to providing interpretable and consistent results.23 In agglomerative hierarchical clustering, each variable is initially considered its own cluster, then variables are joined together with their closest or most similar neighboring cluster (as measured by the Euclidean distance) until all clusters become one. A combination of dendrograms (graphical representation of the clustering process and the distance between clusters) and stopping rules (pseudo F and pseudo T-squared) were used to determine the ideal number of clusters from the possible solutions provided.23, 24 We examined cluster solutions ranging from 2 to 5 clusters, comparing the results of each in order to identify the ideal number of clusters, which was defined as the cluster solution corresponding to a local maximum for the pseudo F and local minimum for the pseudo T-squared.
Once symptom clusters were identified, we looked for associations between the symptom clusters and sociodemographic and clinical variables by assigning participants into symptom cluster groups. To do this, we dichotomized the symptom variables: if a symptom was rated no (0), very little (1), or a little (2) it was dichotomized as no (none/minimal), whereas if a symptom was rated a fair amount (3), a lot (4), or a great deal (5) it was dichotomized as yes. Next, participants were divided into three groups for each symptom cluster, those with: 1) all the symptoms in the cluster, 2) some of the symptoms in the cluster, and 3) none of the symptoms in the cluster. We then compared characteristics of the individuals within each group (all/some/none) for each of the identified symptom clusters. Comparisons were made with Fisher’s exact or chi-square tests for categorical variables and Kruskal-Wallis one-way analysis of variance for continuous and ordinal variables. Because of the descriptive nature of this work, we compared a broad range of sociodemographic and clinical characteristics and a p-value of < 0.05 was considered statistically significant.
Next, we conducted two Poisson regression analyses to determine the association between 1) symptom clusters and AF-related hospitalizations, and 2) symptom clusters and AF-related ED visits. Sociodemographic and clinical variables that were significant when comparing characteristics between individuals with none/some/all symptoms in a cluster were entered into our adjusted analyses as independent variables. Additionally, potential confounders were included in the adjusted models if they were known a priori to be associated with healthcare utilization. Variables were retained in the models if they changed the strength of the association between the symptom clusters and the response variable by more than 10%.
Results
Sample Characteristics
The 1,501 participants in this study were predominantly Caucasian (96%) and primarily male (67%). The age of participants ranged from 18.1 and 88.5 years (M=58.4 years; SD=12.2; Table 1). Exercise intolerance was the most common symptom, affecting 42% of participants, followed by shortness of breath with activity (40%) and palpitations (33%). The sample had mostly paroxysmal (51%) or persistent (42.5%) AF, with only a small portion having permanent AF (6.5%). Average history of AF was 4.5 (SD 5.8) years. Over half the sample was on an anti-arrhythmic medication (55%) and/or had a prior catheter or surgical ablation (52%).
Table 1:
Demographic and Clinical Profile of Participants
| All (N=1,501) |
Female (N=497, 33%) |
Male (N=1,004, 67%) |
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age (years) | 58.4 | (12.2) | 60.9 | (12.8) | 57.1 | (11.7) |
| History of AF (years) | 4.5 | (5.8) | 4.3 | (5.3) | 4.6 | (6.0) |
| Body Mass Index | 31.1 | (6.6) | 30.8 | (7.5) | 31.2 | (6.1) |
| CHADS2 score | 1.1 | (1.1) | 1.2 | (1.2) | 1.1 | (1.1) |
| Left Atrial Diameter (mm) | 42 | (7.8) | 43.1 | (7.7) | 39.8 | (7.6) |
| Left Ventricular Ejection Fraction | 55.3 | (10.1) | 57.4 | (9.3) | 54.2 | (10.3) |
| N | % | N | % | N | % | |
| Ethnicity | ||||||
| Caucasian | 1,436 | (95.7) | 473 | (95.2) | 963 | (95.9) |
| Asian | 4 | (0.3) | 0 | (0) | 4 | (0.4) |
| Black | 53 | (3.5) | 23 | (4.6) | 30 | (3) |
| Hispanic | 6 | (0.4) | 1 | (0.2) | 5 | (0.5) |
| Native American | 2 | (0.1) | 0 | (0) | 2 | (0.2) |
| AF Sub-Type | ||||||
| Paroxysmal | 765 | (51.1) | 308 | (62.1) | 457 | (45.6) |
| Persistent | 636 | (42.5) | 164 | (33.1) | 472 | (47.1) |
| Permanent | 97 | (6.5) | 24 | (4.8) | 73 | (7.3) |
| Heart Failure | 216 | (14.4) | 69 | (13.9) | 147 | (14.7) |
| Hypertension | 927 | (62.0) | 313 | (63.1) | 614 | (61.5) |
| Coronary Artery Disease | 317 | (21.3) | 73 | (14.8) | 244 | (24.5) |
| Valve disease | 391 | (26.8) | 165 | (34.0) | 226 | (23.2) |
| History of AF ablation | 771 | (52.4) | 253 | (52.1) | 518 | (52.5) |
| History of Cardiac Bypass Surgery | 109 | (7.3) | 14 | (2.9) | 95 | (9.5) |
| Digoxin | 213 | (14.4) | 78 | (16.0) | 135 | (13.6) |
| Calcium Channel Blocker | 468 | (31.6) | 165 | (33.7) | 303 | (30.5) |
| Beta Blocker | 730 | (49.2) | 241 | (49.2) | 489 | (49.2) |
| Anti Lipidemic | 630 | (42.5) | 191 | (39.1) | 439 | (44.1) |
| Ace/Angiotensin Blocker | 591 | (39.8) | 187 | (38.2) | 404 | (40.6) |
| Anti-Arrhythmic | 817 | (55.1) | 274 | (56.0) | 543 | (54.6) |
Symptom Clusters
A three-cluster solution was indicated as the optimal solution based on the dendrogram, a maximum pseudo F of 2.2, and a minimum pseudo T-squared of 1.2 (Figure 1). One of the clusters in the three-cluster solution consisted of a single symptom (palpitations), and therefore did not meet our definition of a symptom cluster (two or more co-occurring symptoms). Palpitations did not cluster with other symptoms according to our a priori definition of a symptom cluster. However, because palpitations likely have clinical significance, we compared demographic/clinical characteristics and healthcare utilization in participants with and without palpitations so that this symptom would not be excluded from the remainder of our analysis. We labeled the two clusters that met our definition of a symptom cluster the Weary cluster (fatigue at rest, shortness of breath at rest, chest pain, and dizziness) and the Exertional cluster (shortness of breath with activity and exercise intolerance). The Exertional symptom cluster was the most common, with all symptoms present in 33% (n=491) of participants. The Weary cluster was uncommon, with all symptoms present in only 3.7% (n=56) of participants. Palpitations effected 33% (n=494) of the sample (Table 2). There was significant co-occurrence of the two clusters, with 51 of the 56 participants who had the Weary cluster also having the Exertional cluster (Table 2). Palpitations had some degree of co-occurrence with both clusters: 268 out of the 491 participants who had the Exertional cluster also experienced palpitations.
Figure 1:
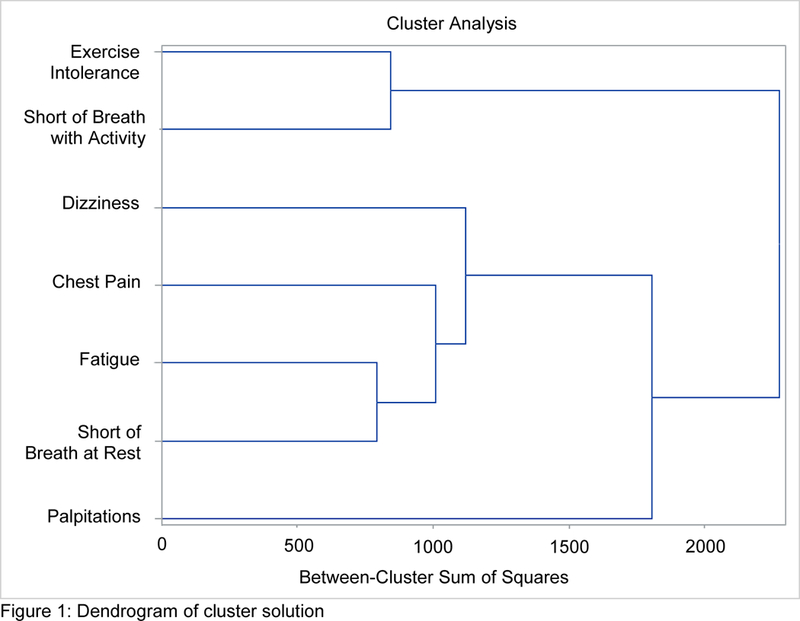
Dendrogram of cluster solution
Table 2:
Cluster Co-occurrence
| Palpitations | Weary | Exertional | |
|---|---|---|---|
| Palpitations | 494 (32.9%) | 47 (3.1%) | 268 (17.8%) |
| Weary | 56 (3.7%) | 51 (3.4%) | |
| Exertional | 491 (32.7%) |
Characteristics by Symptom Cluster Membership
Several sociodemographic and clinical characteristics varied by cluster membership. Women comprised the greatest proportion of the group that had all symptoms in the Weary cluster (52% women versus 48% men, p<0.001), even though the overall sample was two thirds male. Statistically significant differences in ethnicity, body mass index, coronary disease, heart failure, current use of anti-arrhythmic medication, and history of ablation were also apparent (Table 3).
Table 3:
Demographic and Clinical Characteristics by Cluster Membership
| Weary | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None of the Symptoms N=892 |
Some of the Symptoms N=553 |
All of the Symptoms N=56 |
p | ||||||||
| Characteristic | N | % | N | % | N | % | |||||
| Gender | <0.001 | ||||||||||
| Male | 664 | (74) | 313 | (57) | 27 | (48) | |||||
| Female | 228 | (26) | 240 | (43) | 29 | (52) | |||||
| Caucasian | 851 | (95) | 535 | (97) | 50 | (89) | 0.03 | ||||
| AF Sub-Type | 0.49 | ||||||||||
| Paroxysmal | 457 | (51) | 284 | (51) | 24 | (43) | |||||
| Persistent | 371 | (42) | 235 | (43) | 30 | (54) | |||||
| Permanent | 62 | (7) | 33 | (6) | 2 | (4) | |||||
| Coronary Disease | 156 | (18) | 148 | (27) | 13 | (23) | <0.001 | ||||
| Heart Failure | 99 | (11) | 101 | (18) | 16 | (29) | <0.001 | ||||
| History of Ablation | 417 | (48) | 313 | (58) | 41 | (75) | <0.001 | ||||
| Anti-Arrhythmic | 435 | (49) | 344 | (63) | 38 | (67) | <0.001 | ||||
| M | SD | M | SD | M | SD | ||||||
| Age (years) | 58.2 | (12.4) | 58.9 | (12) | 55.8 | (10.1) | 0.09 | ||||
| History of AF (years) | 4.6 | (6.1) | 4.3 | (5.3) | 4.1 | (4.4) | 0.95 | ||||
| Body Mass Index | 30.5 | (6.2) | 31.8 | (7.1) | 32.4 | (7.2) | 0.003 | ||||
| LVEF | 55.6 | (9.9) | 54.7 | (10.5) | 54.8 | (9.8) | 0.24 | ||||
| Exertional | |||||||||||
|
None of the Symptoms N=770 |
Some of the Symptoms N=240 |
All of the Symptoms N=491 |
p | ||||||||
| Characteristic | N | % | N | % | N | % | |||||
| Gender | <0.001 | ||||||||||
| Male | 569 | (74) | 146 | (61) | 289 | (59) | |||||
| Female | 201 | (26) | 94 | (39) | 202 | (41) | |||||
| Caucasian | 734 | (95) | 231 | (96) | 471 | (96) | 0.78 | ||||
| AF Sub-Type | <0.001 | ||||||||||
| Paroxysmal | 445 | (58) | 120 | (50) | 200 | (41) | |||||
| Persistent | 272 | (35) | 107 | (45) | 257 | (52) | |||||
| Permanent | 50 | (7) | 13 | (5) | 34 | (7) | |||||
| Coronary Disease | 121 | (16) | 59 | (25) | 137 | (28) | <0.001 | ||||
| Heart Failure | 65 | (8) | 36 | (15) | 115 | (23) | <0.001 | ||||
| History of Ablation | 334 | (44) | 135 | (58) | 302 | (63) | <0.001 | ||||
| Anti-Arrhythmic | 375 | (49) | 145 | (61) | 297 | (62) | <0.001 | ||||
| M | SD | M | SD | M | SD | ||||||
| Age (years) | 57.5 | (12.5) | 58.9 | (12.1) | 59.5 | (11.8) | 0.01 | ||||
| History of AF (years) | 4.6 | (6.2) | 4.6 | (5.6) | 4.3 | (5.1) | 0.89 | ||||
| Body Mass Index | 30 | (6) | 30.8 | (6.5) | 32.7 | (7.2) | <0.001 | ||||
| LVEF | 55.9 | (9.1) | 55.4 | (10.4) | 54.2 | (11.3) | 0.03 | ||||
| Palpitations | |||||||||||
|
No/Mild N=1007 |
Yes N=494 |
p | |||||||||
| Characteristic | N | % | N | % | |||||||
| Gender | <0.001 | ||||||||||
| Male | 731 | (73) | 273 | (55) | |||||||
| Female | 276 | (27) | 221 | (45) | |||||||
| Caucasian | 962 | (96) | 474 | (96) | 0.71 | ||||||
| AF Sub-Type | <0.001 | ||||||||||
| Paroxysmal | 479 | (48) | 286 | (58) | |||||||
| Persistent | 441 | (44) | 195 | (39) | |||||||
| Permanent | 84 | (8) | 13 | (3) | |||||||
| Coronary Disease | 218 | (22) | 99 | (20) | 0.47 | ||||||
| Heart Failure | 152 | (15) | 64 | (13) | 0.26 | ||||||
| History of Ablation | 464 | (47) | 307 | (63) | <0.001 | ||||||
| Anti-Arrhythmic | 498 | (50) | 319 | (66) | <0.001 | ||||||
| M | SD | M | SD | ||||||||
| Age (years) | 59.0 | (12.2) | 57.2 | (12.3) | 0.002 | ||||||
| History of AF (years) | 4.48 | (6) | 4.55 | (5.2) | 0.07 | ||||||
| Body Mass Index | 31.1 | (6.7) | 31.0 | (6.4) | 0.86 | ||||||
| LVEF | 55.8 | (10.4) | 56.2 | (9.4) | 0.07 | ||||||
Statistically significant p-values (p<0.05) are shown in bold. Data are mean (standard deviation) or number of patients (%). LVEF: left ventricular ejection fraction
For the Exertional cluster, women were again more likely than men to have all of the symptoms in the cluster. Among the group with all Exertional symptoms, 59% were male and 41% were female (p<0.001): because the overall sample was two thirds male, this indicates that compared to men, a greater proportion of the women in our sample have the Exertional cluster of symptoms. Younger individuals were less likely to experience Exertional cluster symptoms (57.5 years versus 59.5 years, p=0.01). Individuals with persistent AF were most likely to experience all the symptoms in the Exertional cluster. Additional sociodemographic and clinical factors that showed statistically significant variance by cluster membership included body mass index, left ventricular ejection fraction, coronary disease, heart failure, current use of anti-arrhythmic medication, and history of ablation (Table 3).
Statistically significant differences were also evident related to the presence of palpitations. Participants with palpitations were an average of about 2 years younger than individuals without palpitations (57.2 years versus 59 years, p=0.002). Palpitations were more likely in women, individuals with paroxysmal AF, on anti-arrhythmic medications, and with a history of ablation (Table 3).
Association between Symptom Clusters and Healthcare Utilization
Emergency department utilization.
In unadjusted analyses, experiencing all the symptoms in the Weary cluster was associated with triple the rate of ED utilization (incident rate ratio/IRR 3.6, p<0.001, Table 4), while having all the symptoms in the Exertional cluster was associated with over one and a half times the rate of ED utilization (IRR 1.6, p<0.001). In the adjusted model, the Exertional cluster no longer had a statistically significant association with ED utilization. Experiencing all the symptoms in the Weary cluster had the strongest association with ED utilization of all variables in the model, with nearly triple the rate of ED utilization compared to individuals with none of the Weary symptoms (IRR 2.8, p<0.001). Although palpitations did not cluster with other symptoms, it was associated with a slightly increased rate of ED utilization in the adjusted model (IRR 1.2, p=0.04).
Table 4:
Healthcare Utilization by Cluster Membership
| Emergency Department | Hospitalizations | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted** | |||||
| Weary Cluster | IRR | p-value | IRR | p-value | IRR | p-value | IRR | p-value |
| None | (ref) | (ref) | (ref) | (ref) | ||||
| Some | 1.66 | <0.001 | 1.45 | <0.001 | 1.72 | <0.001 | 1.35 | 0.001 |
| All | 3.64 | <0.001 | 2.83 | <0.001 | 2.79 | <0.001 | 1.92 | <0.001 |
| Exertional Cluster | ||||||||
| None | (ref) | (ref) | (ref) | (ref) | ||||
| Some | 1.25 | 0.01 | 0.96 | 0.72 | 1.31 | 0.002 | 0.97 | 0.74 |
| All | 1.59 | <0.001 | 0.96 | 0.66 | 1.72 | <0.001 | 0.98 | 0.85 |
| Palpitations | ||||||||
| No | (ref) | (ref) | (ref) | (ref) | ||||
| Yes | 1.86 | <0.001 | 1.18 | 0.04 | 1.74 | <0.001 | 1.27 | 0.002 |
adjusted for gender, age, AF type, history of ablation, current AAD, heart failure, body mass index, coronary disease, left ventricular ejection fraction, history of AF
adjusted for gender, age, ethnicity, AF type, history of ablation, current AAD, heart failure, body mass index, coronary disease, left ventricular ejection fraction, history of AF
Hospitalizations.
Experiencing all the symptoms in the Weary cluster nearly tripled the rate of hospitalizations in unadjusted analyses (IRR 2.8, p<0.001, Table 4), whereas having all symptoms in the Exertional cluster corresponded with more than one and a half times the rate of hospitalizations (IRR 1.7, p<0.001). In the adjusted model, the Exertional cluster no longer had a statistically significant association with hospitalizations (IRR 0.98, p=0.85). Among the retained variables, having all symptoms in the Weary cluster was the most strongly associated with hospitalizations, resulting in almost twice the rate of hospitalizations compared to individuals with none of the Weary symptoms (IRR 1.9, p<0.001). Palpitations also increased the rate of hospitalizations in the adjusted model (IRR 1.3, p=0.002).
Discussion
We identified two AF-specific symptom clusters: The Weary cluster (fatigue at rest, shortness of breath at rest, chest pain, and dizziness) and the Exertional cluster (shortness of breath with activity and exercise intolerance). Experiencing all symptoms in the Weary cluster was associated with a significantly increased rate of both ED utilization and hospitalization. These findings are comparable with prior research which shows that severe symptoms (defined as symptoms that effect daily activities) are a major predictor of incident hospitalizations in patients with AF.6 Our findings add to the body of literature, indicating that the presence of specific symptoms is associated with an increased rate of ED visits and hospitalizations. Recent research indicates that individuals with AF symptoms that are readily attributable to cardiac causes are more likely to seek treatment in a timely manner (<24 hours),25 offering a logical explanation as to why the Weary symptom cluster had the strongest associated with ED utilization.
The clusters identified in this study differ from the previously reported AF-specific symptom clusters identified using participants in the SAFETY trial.11, 26 The clusters identified in SAFETY participants included a Vagal cluster (nausea and diaphoresis), Tired cluster (fatigue, weakness, syncope/dizziness, and dyspnea), and Heart cluster (palpitations and chest pain).11 The most similarity between studies occurs with the Tired cluster and our Weary cluster. Differences between the VAFR and SAFETY recruitment strategies, inclusion criteria, and approach to symptom measurement likely account for the differences between the symptom clusters found in these studies. Differences in the sample characteristics of VAFR and SAFETY are also worth noting. First, the mean age of our VAFR sample was 58.4 years, compared to a mean age of 72 years for SAFETY participants. Second, 88% of SAFETY participants had persistent AF and 3% had paroxysmal AF, compared to 43% and 51% respectively for VAFR. In the SAFETY cluster analysis, subjects with both the Heart cluster symptoms (chest pain and palpitations) were about 4 years younger than those with none of the symptoms,11 which is similar to our finding that individuals with palpitations were approximately 2 years younger than those without. In the SAFETY cluster analysis, palpitations clustered with chest pain,11 whereas in the present study palpitations stood alone. It is possible this difference in clustering for palpitations is related to differences in age, AF type, comorbidity profiles, or clinical and pharmacological management between the two study populations.
Although studies examining symptom clusters in AF are rare, several studies have examined symptom clusters in adults with heart failure and coronary artery disease.10 Heart failure symptoms are often split into physical versus emotional clusters.10 Symptom clusters labeled as ‘weary’ have been reported in patients with heart failure and ischemic coronary disease.10 The exact symptoms in ‘weary’ and ‘physical’ clusters varies slightly from study to study, but tend to include fatigue, shortness of breath/dyspnea with exertion, and difficulty sleeping. Compared to other cardiovascular conditions, AF symptom clusters have the most similarities with the weary and/or physical symptom clusters,10, 11 evidenced by shortness of breath and fatigue clustering together. For example, Lindgren27 described a weary symptom cluster among adults with ischemic heart disease that included shortness of breath, fatigue, pain, and difficulty sleeping. It is important to note that clustering of fatigue and shortness of breath in AF symptom clusters occurred both in our study, which included individuals with heart failure, and in SAFETY,11 which excluded individuals with heart failure. Noting the similarities in physical symptom clustering between heart failure and AF, future prospective studies of AF symptom clusters should include psychological symptoms and comorbidities to determine if similarities also exist regarding emotional symptom clusters.
Several statistically significant differences were noted in the sociodemographic and clinical characteristics of individuals based on symptom cluster group membership. Individuals with all of the Weary and/or Exertional symptom clusters were more likely to be female, have an elevated BMI, have heart failure and/or coronary disease, be on anti-arrhythmic therapy, and have a history of ablation. Consistent with clinical recommendations5 our findings indicate that rhythm control strategies (anti-arrhythmic medications and ablation) were more commonly used for individuals with significant symptom burden.
Our finding that women are more likely to experience palpitations, the Weary, and the Exertional symptom clusters is consistent with prior AF symptom cluster research, which similarly found that women were more likely to experience a cluster of palpitations and chest pain.11 In fact, most AF symptom literature indicates that women report more frequent and severe symptoms than men.28, 29
Obesity is a known risk factor for increased AF burden and symptom severity and our findings provide further evidence of the relationship between obesity and AF symptoms.30, 31 The association between obesity and AF symptoms may be confounded by physical inactivity and depression32 indicating two modifiable factors that could be targeted in interventions aimed at improving AF symptom management.
AF and heart failure have many symptoms in common (e.g. shortness of breath, fatigue), so it is not surprising that individuals with heart failure were more likely to experience both symptom clusters. Concomitant heart failure and AF may further exacerbate each other due to the effects of each on hemodynamic function, making symptomatic management of both conditions more challenging. Pre-existing heart failure is a primary predictor for hospitalization among individuals with AF (hazard ratio 1.57), indicating individuals with symptomatic AF and heart failure may benefit the most from interventions designed to improve AF symptom management.6
Symptom cluster evaluation is an important contribution cardiovascular nurses can make to clinical practice and symptom management. Several studies in cardiovascular populations (e.g. heart failure and acute coronary syndrome) have demonstrated an association between specific clusters of symptoms and health outcomes such as mortality,14, 33 event-free survival,13, 34 and major adverse cardiac events.15 Our findings reveal that individuals with the Exertional symptoms were older and had persistent AF. This information is useful for clinical evaluation and decision making, suggesting the importance of careful symptom assessment in this subset of the AF population for intermittent and vague symptoms, which prior research indicates are often attributed to other causes (e.g. aging, deconditioning).25 Early and accurate identification of AF symptoms has the potential to lead to improved health outcomes. Nursing assessment for specific symptom profiles may be particularly important given recent research that indicates individuals who experience vague or intermittent AF symptoms are less likely to seek treatment in a timely manner.25
To date, symptom management for AF has largely focused on rate and/or rhythm control strategies via medical or procedural management. Studies of self-care strategies to improve symptom management are largely lacking, and the few studies published are in the early stages of intervention development.35, 36 Self-care is defined as the decision-making processes that individuals use to maintain health and manage illness, with symptom perception acknowledged as having a profound impact on self-care outcomes.37 Health-promoting lifestyle choices are an important component of self-care (e.g. nutritious diet, exercise), which are beginning to be explored as options for AF symptom management due to the deleterious effect of obesity on AF burden and symptom management.30, 31 Alternative therapies (e.g. yoga and biofeedback) have demonstrated symptom reduction in adults with AF, although sample sizes are small and confirmatory studies are needed.38, 39 Nurse-led integrated care is another approach with the potential to improve AF symptom management.40 Effective self-care for chronic conditions requires an individual monitor signs and symptoms and know how to appropriately respond when symptoms occur.37 As such, interventions that teach coping strategies, employ support groups, or use cognitive-behavioral therapy could also potentially improve symptom related self-care by addressing the neurocognitive variables that influence AF symptom perception. Additional research is warranted to explore the effect of self-care and nursing interventions on AF symptoms and symptom clusters.
Limitations
There are several important limitations to our study. The most significant limitation of our study is the lack of psychological comorbidities (e.g. depression, anxiety) and symptoms (e.g. worry, fear). Psychological comorbidities influence both the number and severity of AF symptoms, and psychological symptoms are likely important components of symptom clusters, but we were unable to examine this possibility since they were not measured as part of the registry. Future prospective studies of AF symptom clusters should include psychological symptoms and covariates. Second, our sample was primarily Caucasian and male, however AF is more common in this demographic and as such does not necessarily limit the generalizability of our findings. Third, because we used a deidentified version of the VAFR data, we do not know the dates of ablations. Finally, the healthcare utilization variables were self-reported. Future studies using medical record review or claims data should be conducted to confirm our findings.
Conclusion
This study provides evidence of two AF-specific symptom clusters. Several sociodemographic and clinical characteristics varied by symptom cluster group membership. The Weary cluster was associated with the highest rate of healthcare utilization. Our findings indicate that specific symptom profiles are associated with health outcomes. Additional prospective research is warranted to determine if symptom clusters share common underlying biological or behavioral mechanisms, and to further elucidate the role of AF symptom clusters in relation to patient centered-health outcomes.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball J, Carrington MJ, McMurray JJV, et al. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol 2013. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation 2016. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg BA, Kim S, Fonarow GC, et al. Drivers of hospitalization for patients with atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rienstra M, Lubitz SA, Mahida S, et al. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears SF, Serber ER, Alvarez LG, et al. Understanding atrial symptom reports: objective versus subjective predictors. Pacing Clin Electrophysiol 2005. [DOI] [PubMed] [Google Scholar]
- 9.Riegel B, Dickson VV, Lee CS, et al. A mixed methods study of symptom perception in patients with chronic heart failure. Heart Lung 2018. DOI: 10.1016/j.hrtlng.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVon HA, Vuckovic K, Ryan CJ, et al. Systematic review of symptom clusters in cardiovascular disease. Eur J Cardiovasc Nurs 2017. DOI: 10.1177/1474515116642594. [DOI] [PubMed] [Google Scholar]
- 11.Streur M, Ratcliffe SJ, Ball J, et al. Symptom Clusters in Adults with Chronic Atrial Fibrillation. J Cardiovasc Nurs 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, McGuire DB, Tulman L, et al. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs 2005. [DOI] [PubMed] [Google Scholar]
- 13.Song EK, Moser DK, Rayens MK, et al. Symptom clusters predict event-free survival in patients with heart failure. J Cardiovasc Nurs 2010. DOI: 10.1097/JCN.0b013e3181cfbcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang SY, Ahn YG and Jeong MH. Atypical symptom cluster predicts a higher mortality in patients with first-time acute myocardial infarction. Korean Circ J 2012. DOI: 10.4070/kcj.2012.42.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang SY and Kim J. Cluster dyads of risk factors and symptoms are associated with major adverse cardiac events in patients with acute myocardial infarction. Int J Nurs Pract 2015. DOI: 10.1111/ijn.12241. [DOI] [PubMed] [Google Scholar]
- 16.Darbar D, Motsinger AA, Ritchie MD, et al. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart Rhythm 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001. [DOI] [PubMed] [Google Scholar]
- 19.Pamukcu B, Lip GYH and Lane DA. Simplifying stroke risk stratification in atrial fibrillation patients: implications of the CHA2DS2-VASc risk stratification scores. Age Ageing 2010. DOI: 10.1093/ageing/afq059. [DOI] [PubMed] [Google Scholar]
- 20.Dorian P, Burk C, Mullin CM, et al. Interpreting changes in quality of life in atrial fibrillation: how much change is meaningful? Am Heart J 2013. [DOI] [PubMed] [Google Scholar]
- 21.Dorian P, Paquette M, Newman D, et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J 2002. [DOI] [PubMed] [Google Scholar]
- 22.Dorian P, Guerra PG, Kerr CR, et al. Validation of a new simple scale to measure symptoms in atrial fibrillation: the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Circ Arrhythm Electrophysiol 2009. [DOI] [PubMed] [Google Scholar]
- 23.Everitt B, Landau S and Leese M. Cluster analysis. 4. ed. ed. New York: Oxford University Press, 2001. [Google Scholar]
- 24.Milligan GW and Cooper MC. An examination of procedures for determining the number of clusters in a data set. Psychometrika 1985. DOI: 10.1007/BF02294245. [Google Scholar]
- 25.McCabe PJ, Rhudy LM, Chamberlain AM, et al. Fatigue, dyspnea, and intermittent symptoms are associated with treatment-seeking delay for symptoms of atrial fibrillation before diagnosis. Eur J Cardiovasc Nurs 2016. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Ball J, Horowitz JD, et al. Standard versus atrial fibrillation-specific management strategy (SAFETY) to reduce recurrent admission and prolong survival: pragmatic, multicentre, randomised controlled trial. Lancet 2015. [DOI] [PubMed] [Google Scholar]
- 27.Lindgren TG, Fukuoka Y, Rankin SH, et al. Cluster analysis of elderly cardiac patients’ prehospital symptomatology. Nurs Res 2008. DOI: 10.1097/01.NNR.0000280654.50642.1a. [DOI] [PubMed] [Google Scholar]
- 28.Thompson TS, Barksdale DJ, Sears SF, et al. The effect of anxiety and depression on symptoms attributed to atrial fibrillation. Pacing Clin Electrophysiol 2014. [DOI] [PubMed] [Google Scholar]
- 29.Paquette M, Roy D, Talajic M, et al. Role of gender and personality on quality-of-life impairment in intermittent atrial fibrillation. Am J Cardiol 2000. [DOI] [PubMed] [Google Scholar]
- 30.Pathak RK, Elliott A, Middeldorp ME, et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals with Atrial Fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol 2015. [DOI] [PubMed] [Google Scholar]
- 31.Pathak RK, Middeldorp ME, Meredith M, et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol 2015. [DOI] [PubMed] [Google Scholar]
- 32.Garimella RS, Sears SF and Gehi AK. Depression and Physical Inactivity as Confounding the Effect of Obesity on Atrial Fibrillation. Am J Cardiol 2016. [DOI] [PubMed] [Google Scholar]
- 33.Riegel B, Hanlon AL, McKinley S, et al. Differences in mortality in acute coronary syndrome symptom clusters. Am Heart J 2010. DOI: 10.1016/j.ahj.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KS, Song EK, Lennie TA, et al. Symptom clusters in men and women with heart failure and their impact on cardiac event-free survival. J Cardiovasc Nurs 2010. DOI: 10.1097/JCN.0b013e3181cfbb88. [DOI] [PubMed] [Google Scholar]
- 35.McCabe PJ, Douglas KV, Barton DL, et al. Feasibility Testing of the Alert for AFib Intervention. West J Nurs Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guhl EN, Schlusser CL, Henault LE, et al. Rationale and design of the Atrial Fibrillation health Literacy Information Technology Trial: (AF-LITT). Contemp Clin Trials 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riegel B, Jaarsma T and Strömberg A. A middle-range theory of self-care of chronic illness. ANS Adv Nurs Sci 2012. [DOI] [PubMed] [Google Scholar]
- 38.Lakkireddy D, Atkins D, Pillarisetti J, et al. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA My Heart Study. J Am Coll Cardiol 2013. [DOI] [PubMed] [Google Scholar]
- 39.Kanmanthareddy A, Reddy M, Ponnaganti G, et al. Alternative medicine in atrial fibrillation treatment-Yoga, acupuncture, biofeedback and more. J Thorac Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallagher C, Elliott AD, Wong CX, et al. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart 2017. DOI: 10.1136/heartjnl-2016-310952. [DOI] [PubMed] [Google Scholar]


