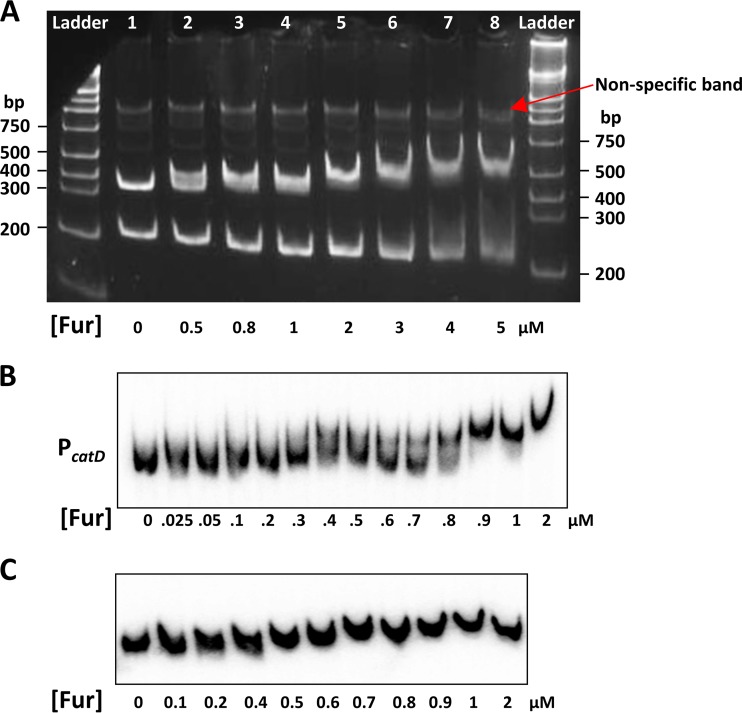
FIG 4.
Fur specifically binds to the promoter region of catDE in vitro. An electrophoretic mobility shift assay (EMSA) was carried out using two sets of DNA probes. (A) The promoter region of catD and part of its open reading frame (–155 to +386 bp) were amplified by PCR and digested using HindIII, generating two fragments (lane 1): a 323-bp band (–155 to +168 bp; middle band) encompassing the promoter region and a 218-bp fragment located inside catD (+169 to +386 bp; bottom band), which serves as a negative control. A significant DNA shift (∼50%) was observed with the lowest concentration of Fur (0.5 µM) for the middle band, whereas no evident DNA shift was observed with up to 3 µM of Fur protein for the bottom band. The red arrow indicates a nonspecific band from PCR amplification. About 180 ng of DNA probe was used for each reaction. The 5% native polyacrylamide gel was stained with ethidium bromide. (B) To determine the biochemical affinity of Fur binding to the operator site of the catDE operon, the promoter region (–121 to +76 bp) was PCR amplified and labeled at the 5′-ends with [γ-32P]-ATP. The Kd value was calculated using GraphPad Prism 5 based on three independent experiments. (C) The DNA fragment (within the open reading frame of catD; +137 to +278 bp) was used as a negative control to evaluate nonspecific binding by Fur. No evident DNA shift was observed with up to 2 µM of Fur protein tested. Approximately 1 fmol of labeled DNA probe was used in each reaction shown in panels B and C.