FIG 1.
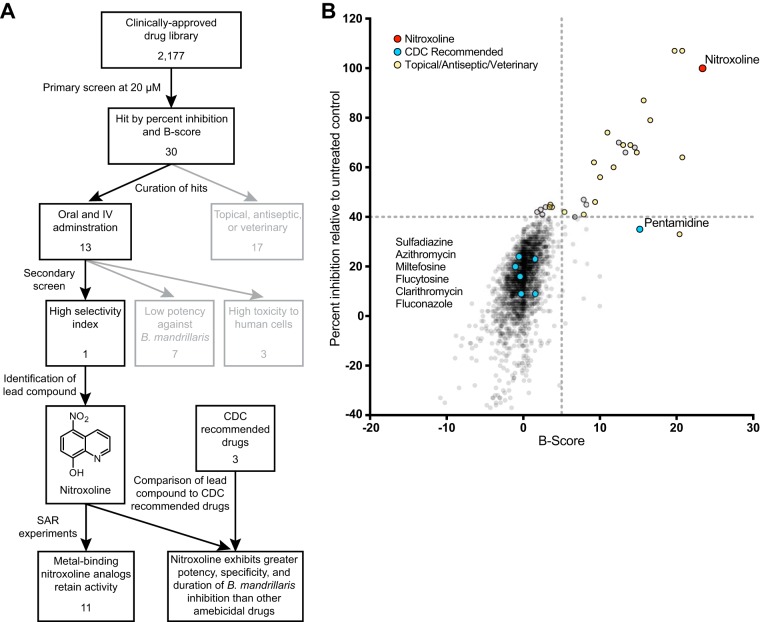
(A) Workflow for screening of clinically approved compounds for in vitro activity against B. mandrillaris. A primary screen of 2,177 clinically approved compounds yielded 30 hits meeting the percent inhibition and B-score criteria, among which only 13 candidates were available for oral or intravenous (IV) administration (see panel B). Secondary screening identified only one novel lead compound, nitroxoline, which displayed high selectivity for inhibition of B. mandrillaris viability (Table S1C). Structure-activity relationship (SAR) experiments showed that 11 of 12 nitroxoline analogs tested with potential metal binding domains remained active against B. mandrillaris, suggesting that metal binding plays a role in the mechanism of inhibition by nitroxoline (Fig. 2). Comparison of nitroxoline to three drugs recommended by the CDC for treatment of B. mandrillaris CNS infections (pentamidine isethionate, miltefosine, and azithromycin) indicates that nitroxoline is the most potent and specific inhibitor of B. mandrillaris among the compounds tested (Fig. 3 and 4; see also Table S1). (B) Plot of percent inhibition relative to untreated controls and B-score measured for each compound in a library of 2,177 clinically approved compounds. Raw data used to calculate these values are compiled in Table S1A. Drugs recommended by the CDC for treatment of GAE are highlighted in blue. Drugs that are classified as antiseptic or topical and/or have not been used in humans are shown in yellow. The quinoline antibiotic nitroxoline, which was the top hit identified in this screen, is highlighted in red.