FIG 2.
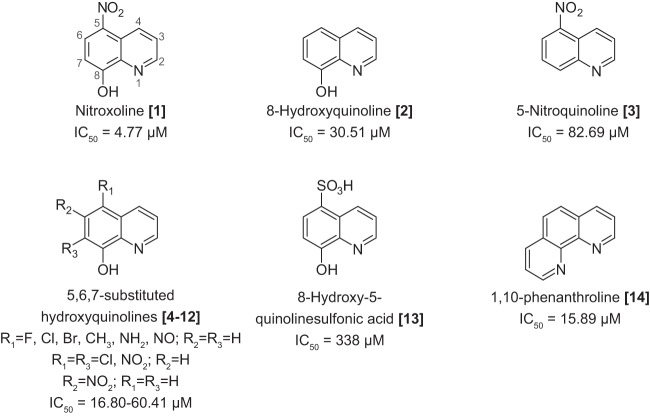
Structure-activity relationship experiments suggest that nitroxoline inhibits B. mandrillaris through a mechanism related to metal binding. Structures and IC50 values are shown for nitroxoline and select analogs; additional compounds are shown in Table S2. Nitroxoline is made up of a quinoline core with a nitro group at the 5 position and a hydroxyl group at the 8 position. Analogs lacking the 8-position hydroxyl group were generally inactive, with IC50 values greater than 80 µM (e.g., 5-nitroquinoline [compound 3]). Twelve nitroxoline analogs with predicted metal binding activity were tested, and of these, 1,10-phenanthroline (compound 14) and 10 of 11 compounds with an 8-position hydroxyl group (e.g., 8-hydroxyquinoline [compound 2]) were active, with IC50 values ranging from 17 to 60 µM. The only inactive analog with an 8-position hydroxyl group was 8-hydroxy-5-quinolinesulfonic acid (compound 13). Variance of the 5-position nitro group reduced potency compared to nitroxoline, but no trend related to aromatic electronic effects is apparent.