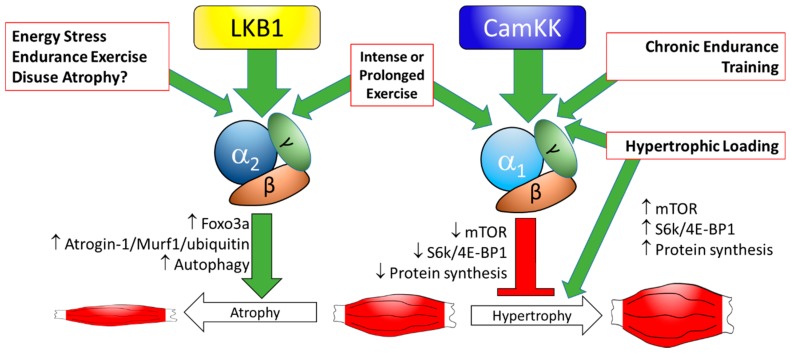
Figure 1.
Proposed regulation of skeletal muscle size by 5’-adenosine monophosphate-activated protein kinase (AMPK). Energy stress (decreased ATP/AMP ratio; as in moderately intensive exercise) predominantly activates AMPKα2 via liver kinase B1 (LKB1), while AMPKα1 is only activated by highly-intense or prolonged exercise. Basal AMPKα1 content and activity is also increased by long-term endurance training, perhaps via Calcium/calmodulin-dependent protein kinase (CamKK) action, or other AMPK kinases. AMPKα2 stimulates catabolic processes by increasing Foxo3a, Atrogin-1 and MuRF-1 expression/activity and increasing autophagy, leading, under certain circumstance, to muscle atrophy, but has little effect on protein anabolism. AMPKα1 impairs mTOR signaling, slows protein synthesis, and blocks hypertrophy. Hypertrophic loading (i.e., resistance exercise) stimulates mechanistic target of rapamycin (mTOR) signaling, protein synthesis, and hypertrophy, but also activates AMPKα1 independent of LKB1 (perhaps via CamKK or other means), limiting the hypertrophic growth. ↑: increase expression or activity; ↓: decreased expression or activity.