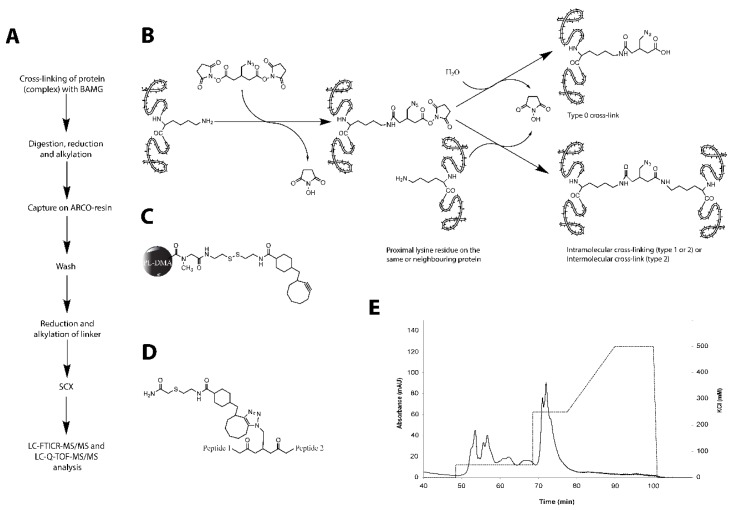
Figure 1.
Overview of the used cross-linking methodology and terminology. (A) experimental work flow. Proteins are incubated with the azide-containing cross-linker bis(succinimidyl)-3-azidomethyl glutarate (BAMG). After cross-linking, proteins are digested and the obtained peptide mixture is incubated with the azide-reactive cyclooctyne (ARCO) resin to capture the cross-linked peptides out of the bulk of unmodified peptides. After cleavage from the resin, enriched peptides are fractionated by strong cation exchange (SCX) chromatography and analyzed by mass spectrometry. (B) structure of BAMG and its reactions with lysine residues in proteins. BAMG can react with a single lysine residue, the other reactive half of the cross-linker becoming either hydrolyzed or reacted with the quenching agent used to stop further cross-linking (type 0 cross-link), or it can react with two proximal lysine residues (type 1 or type 2 cross-linking). A type 1 cross-link occurs between two lysine residues in the same peptide after proteolytic digestion, while a type 2 cross-link connects lysine residues in different peptides. A type 2 cross-link can be formed in the same protein (intramolecular cross-linking) or between two different neighboring proteins (intermolecular cross-linking). BAMG adds 169.1 Da to a type 0 cross-linked peptide and 151.0 Da to type 1 and type 2 cross-linked peptides. (C) ARCO-resin, consisting of a poly-dimethylacrylamide solid support, a disulphide as a cleavable linker, and a cyclooctyne as a reactive group towards azides. Via the strain-promoted azide–alkyne cycloaddition, azide-containing peptides are captured on the resin. (D) enriched type 2 cross-linked peptides. The modification adds 509.2 Da to type 1 and type 2 cross-links and 527.2 Da to type 0 cross-links. (E) SCX chromatogram of enriched peptides. Type 0 and type 1 cross-linked peptides (solid line) elute predominantly at 50 mM KCl (dashed line), while elution of most type 2 cross-links occurs at a higher KCl concentration.