Figure 4.
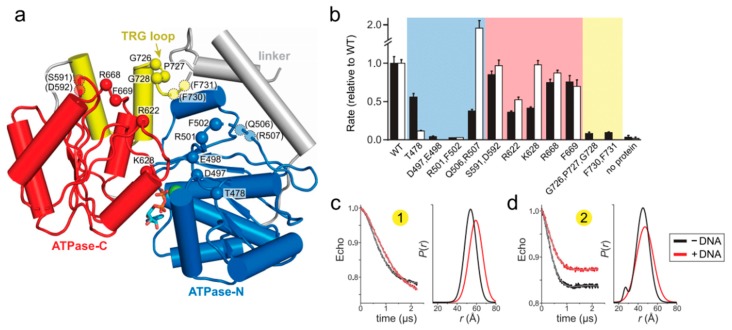
Loops within the TRG motif are essential for DNA-dependent ATP hydrolysis and fork reversal activity. (a) Structure of the ATPase domain (blue and red) with residues lining the putative DNA binding surface shown as Cα spheres. The TRG hairpin and loop are colored yellow. Dashed lines represent disordered regions in the crystal structure. (b) Relative DNA-dependent ATP hydrolysis (black bars) and fork reversal activities (white bars) of alanine mutants. Shading corresponds to the location of each mutant in the structure shown in panel a. Raw data and rates are shown in Figure S3. (c,d) DEER measurements for spin-label pairs 1 (c) and 2 (d) in the TRG loop mutant, G726A P727A G728A. Pairwise time domain data and individual fits of the DEER data are shown on the left and right of each panel, respectively.