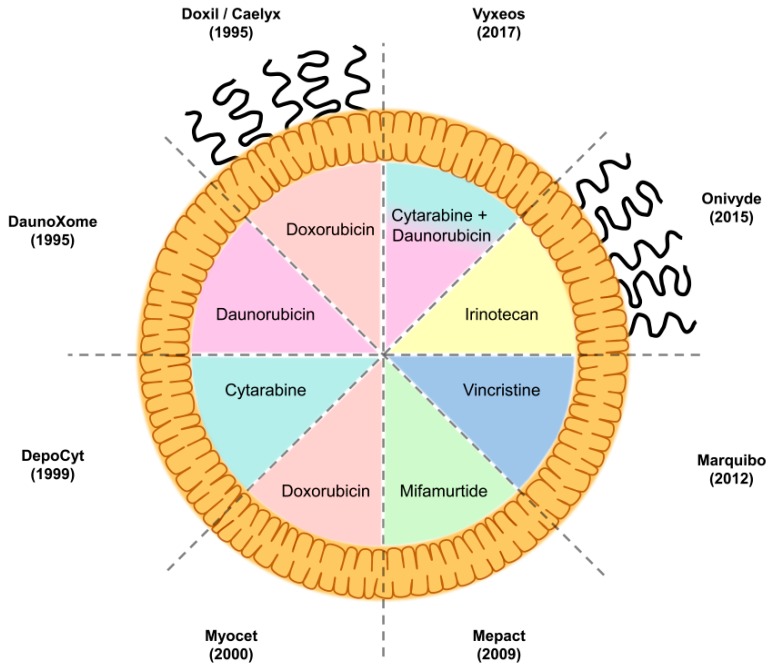
Figure 2.
Liposomal formulations used in the clinic. Commercial name and year of first approval by the authorities is provided for each of the formulations. Loaded drug and decoration of liposomal surface with PEG (in the case of Doxil™/Caelyx™ and Onivyde™) is represented in the schematics. Doxil™/Caelyx™ was the first formulation to receive approval in 1995, and consisted of PEGylated liposomal doxorubicin. Vyxeos™, approved in 2017, is, so far, the last approved liposomal formulation, and it contains a combination of two encapsulated drugs at a fixed 5:1 molar ratio of cytarabine/daunorubicin. Patisiran, a small interfering RNA (siRNA) formulation for treatment of hereditary amyloid transthyretin (ATTR) amyloidosis, will likely be approved in 2018, the year that this review was written.