Abstract
Background: The adverse outcomes of malnutrition on the development of a child are well acknowledged as are the broad variety of contextual factors that may impact child nutritional status. Adequate nutrient intake and the adoption of appropriate water, sanitation and hygiene measures are largely documented for their positive influence on health. Improved sanitation and protection from human feces can significantly lower the incidence of diarrhea and environmental enteropathy. However, the impact of excessive exposure to animal feces on child health is less well documented. Objectives: This study tests the hypothesis that there is a positive association between exposure to animal feces, morbidity and anthropometric outcomes in children under 5 years of age, in Cambodia. It aims to improve insights that can contribute to discerning high-impact policies that promote children can develop to their full potential. Methods: Data for this study was drawn from the third follow-up round of the MyHealth project cohort study that is conducted in six districts of three Cambodian provinces (Phnom Penh, Kratie and Ratanak Kiri). The analysis included a sample of 639 children under 5 years of age. Results: The presence of livestock and more particularly, pigs near the main household dwelling was found a risk factor associated with Giardia duodenalis infection (23%). Giardia duodenalis infection was found to be a protective factor for acute diarrhea, yet, associated with stunting in the univariate model. Conclusions: Preventive measures that protect from extensive exposure to animal feces may be most effective to prevent infection with Giardia duodenalis and consequent stunting, thereby improving the potential for a healthy development in young Cambodian children. The results support the need for cross-sector policy measures that reinforce comprehensive early childhood interventions towards improving nutritional status as part of a wider set of child welfare and development measures.
Keywords: Giardia duodenalis, diarrhea, livestock ownership, feces, stunting
1. Background
Over the last decades, Cambodia has made significant improvements in key health indicators. Yet, stunting in children remains high (32.4% of children below the age of five years, in 2014) and continues to considerably affect their health and development potential [1,2]. Interventions to tackle stunting in children mainly focus on evidence that promotes adequate nutrient intake and the adoption of appropriate water-sanitation-hygiene (WASH) measures to prevent contamination with pathogens from human feces that have been strongly associated with diarrheal illnesses in children [3].
The impact of exposure to animal feces on nutrition status has been little explored [4,5] even exposure to animal feces may be common in low-income countries (LIC) where animals, both domestic and livestock frequently share the household dwelling. Recent literature highlights the importance of zoonotic pathogens on human health. It is estimated that nearly two-thirds of human pathogens (61% of 1415 pathogens known to infect humans) and three quarters of emerging pathogens are of zoonotic origin [6]. Exposure to poultry has been positively associated with an increased risk of respiratory infections in both, adults and young children [7] and a recent meta-analysis that re-examined linkages between exposure to animals and diarrhoea in young children found that 21 out of 27 suitable studies reported significant positive associations [8].
Among the pathogens involved in digestive disorders, certain parasite infections remain a serious threat. Though, studies that explore intestinal protozoal and soil-transmitted helminth infection prevalence in impoverished regions of the world remain limited. Giardia duodenalis (syn. Giardia intestinalis, Giardia lamblia; abbr. G. duodenalis) is found the most common intestinal parasite in humans in developed countries and has a global distribution. In Asia, Africa and Latin America, about 200 million people were identified with symptomatic Giardiasis with about 500,000 new cases reported each year [9]. The presence of Giardia duodenalis has also been noted in domestic animals, and more particularly in livestock, domestic dogs and cats and numerous species of wild mammals [10].
Exposure to animal feces was identified as a potential risk factor for intestinal infections in children and their caregivers in a study by Zambrano [8]. In this study modest evidence related exposure to livestock and animal feces with an increased probability of diarrheal infections. Furthermore, it has been hypothesized that exposure to animal feces may increase high concentrations of bacteria—even non-pathogenic bacteria—in the small intestines and be responsible for chronic subclinical damage and environmental enteric dysfunction (EED) leading to stunting [11,12,13].
This study explores associations between exposure of young children to animal feces and nutritional status with the aim to improve insights that may contribute to discerning policies that promote children to develop to their full potential. We considered exposure to animal feces and nutritional status against a series of co-variables regarding morbidity and socio-economic characteristics. We have chosen Cambodia as setting, because of its high rate of stunting in children under 5 years of age (prevalence 32.4%) [14], limited adoption of good hygiene and nutrition practices and routine presence of livestock at residence allowing for the identification of relevant associations [15,16].
2. Methods
2.1. Study Design and Sampling
Data for this study was drawn from the third follow-up round of the MyHealth project cohort study. The main objective of this project is to collect in-depth data for at least 3 years on health and nutritional status to better inform the government on progresses that can be made with enhanced health monitoring. At baseline, a sample size of 1200 children under 3 years of age per site was calculated required to observe a reduction in child stunting from 32% to 26% over a 3 to 5 years period (with a precision of 3% and a dropout of 20%). Local midwives and village health volunteers provided a list with the names of all children under 3 years of age in the selected areas. This list served as a sampling frame for the sample selection process. Households from children under the age of 3 years were randomly designated to the project using random tables. Data from two selected provinces (Kratie and Ratanak Kiri) were included in this study, data from the capital Phnom Penh was not included due to the low prevalence of domestic and livestock animals at household level.
A sample size of 682 children was calculated required to get a precision of 3% with a type 1 error of 5% using a prevalence of Giardiasis of 20% based on a previous study on G. duodenalis infection in a rural village, in Cambodia [17]. After data collection and cleaning, a final sample size of 639 subjects was available for analysis, allowing to estimate G. duodenalis infection prevalence with a precision of 3.1%. Data was collected through interviews with the mothers of the selected children and a series of anthropometric measures. Some mothers attended the interview but did not attend anthropometric data collection. Information on anthropometric status was available for 593 children in total (Kratie n = 302; Ratanak Kiri n = 291). This latter sample was used for the analysis of malnutrition indicators.
2.2. Study Indicators
Gender, weight, height, and Mid-upper Arm Circumference (MUAC) were recorded for all children. Height was measured by field workers using UNICEF height boards with standing plates and moveable head boards and accuracy to 1 mm. Weight was measured using calibrated digital balances (SECA, Hamburg, Germany) with 100 g precision. MUAC of children was measured using a plastic, colored, insertion tape (marked in millimeters, with cut-off points from red to yellow at 110 mm and from yellow to green at 125 mm; incapable of stretching and unresponsive to temperatures; supplied by UNICEF Copenhagen). We calculated height-for-age Z-scores (HAZ) and weight-for-age Z-scores (WAZ) using WHO 2006 standards for children 0–59 months [17] and WHO Anthro software (version 3.2.2, January 2011, World Health Organization, 1211 Geneva 27, Switzerland). Z-scores < −2 SD for length/height-for-age was defined as stunting while wasting was weight-for-length/height < −2 SD and MUAC below 115 mm [18].
One day before the interview, flasks were given to the mothers included in the study sample in order to collect a stool sample of participating children. The flasks were returned to the interviewers the next day, preserved in a 10% formalin solution and sent for analysis to the Pasteur Institute in Phnom Penh. Stool samples were examined using a coproscopic technique. Simple qualitative direct examination analysis was conducted to highlight the presence of cysts of G. duodenalis (microscopic examination). Giardia infection was categorized as presence/absence of G. duodenalis cysts. Acute diarrhea episodes were measured through maternal recall (three or more loose stools passed in a 24-h period) with at least one episode in the two weeks before the interview.
2.3. Covariates
The main covariates for this study were: type of livestock ownership, sanitation facilities, drinking water source and place where the child usually defecated. Information on these indicators were asked directly to the mother during the interview. The different types of livestock included pig, cow and chicken. Sanitation facilities were categorized into improved and non-improved as per WHO definitions. Improved sanitation facilities included: flush toilets, ventilated improved pit latrines and pit latrines with slabs. Source of drinking water was defined as improved if the household used: piped water, public tap or standpipe, tube well or borehole, protected dug well or spring (public or private), or bottled water in line with the categorization used in the Cambodia Demographic Health Survey (CDHS). Place where the child usually defecated, included: yard, own clothes, diaper, latrine and potty. Yard and own clothes were considered as unsafe defecation places. Child factors, mother education and household wealth index were considered as potential cofounders. Child factors included age in months and gender. Mother education was categorized into three categories: no education/informal schooling, primary education and secondary or more. Household socio-economic status was represented through a wealth index. This index was calculated using the baseline data of the survey. Its calculation was done through principal component analysis with variables on ownership of house and land, housing quality and household assets, as described by Filmer and Pritchett [18] and the first principal component to be divided into quintiles. For this study, the two first quintiles were grouped together as well as the two last ones, leading to an index with three categories: poor, middle class and rich. Finally, as models were fitted on the full sample (grouping the two provinces together) a region indicator variable was systematically included in the models.
2.4. Statistical Analysis
Data management and statistical analysis were done using STATA version 13 (Stata Corp., College Station, TX, USA) and R software version 3.4.0 (Free Software Foundation (FSF), MA, USA). First, descriptive analyses were conducted to examine different background characteristics and distribution factors across the two regions. Associations were examined between G. duodenalis infection and ownership of different types of livestock, WASH factors, age, mother education and wealth index using a multivariate logit model. All selected variables were included in the final model. A focus was made on the association between G. duodenalis infection and three other outcomes (symptoms of diarrhea, HAZ and WHZ), including them as responses and the presence of G. duodenalis as the main independent variable. The binary diarrhea variable was analyzed using a multivariate logit model whereas linear regression models were fitted for the two continuous anthropometric variables. For these three models, all the covariates were considered at the first stage of model building. To build final models parsimonious with control for cofounding effect, the selection among these variables was done using the method as proposed by Hosmer and Lemeshow [19].
In addition to controlling all our models for region and age of the child; the two anthropometric models also had gender, age and age squared included as forced variables. To facilitate comparisons across variables included in all regression models, both, unadjusted (bivariate) and adjusted (multivariate) coefficients were shown. When the distribution of both, the outcome and the included covariate differed considerably between regions (Kratie and Ratanak Kiri), a univariate analysis for this covariate was done adjusting for region. For all analyses, the type I error risk was 0.05.
2.5. Ethical Approval
Ethical approval for the study was obtained from the Cambodia National Ethical Committee for Health Research (117/NECHR). Informed consent was obtained from all participants, with consent obtained from parents or guardians for participating children.
3. Results
3.1. Descriptive Analysis
Table 1, provides an overview of selected sample characteristics. Combining the two regions together (N = 639), a quarter of the children (23.0%) presented G. duodenalis infection. The mean child’s age was 20.6 months, gender distribution was 49.6% boys. Wealth index identified 50.2% of households as poor and 22% as rich. Households owned pigs, cows and chicken at a rate of 39.1%, 39.9% and 14% respectively.
Table 1.
Summary of the selected sample characteristics.
| Kratie | Ratanak Kiri | Overall | p Value † | |
|---|---|---|---|---|
| Full sample | ||||
| N | 378 | 322 | 700 | |
| Child play outside, % (SE) | 91.8 (1.4) | 90.7 (1.6) | 91.3 (1.1) | 0.594 |
| Study sample | ||||
| N | 347 | 292 | 639 | |
| Demographic variable | ||||
| Age in months, mean (SD) | 20.7 (±8.2) | 20.6 (±8.1) | 20.6 (±8.2) | 0.854 |
| Boys, % (SE) | 50.1 (2.7) | 49.0 (2.9) | 49.6 (1.2) | 0.768 |
| Morbidity | ||||
| Giardia Duodenalis, % (SE) | 23.6 (2.3) | 22.3 (2.4) | 23 (1.7) | 0.682 |
| Had diarrhea last 15 days, % (SE) | 7.2 (1.4) | 21.2 (2.4) | 13.6 (1.4) | 0.000 |
| Animal owned | ||||
| Pig, % (SE) | 27.7 (2.4) | 52.7 (2.9) | 39.1 (1.9) | 0.000 |
| Cow, % (SE) | 62.2 (2.6) | 13.4 (2) | 39.9 (1.9) | 0.000 |
| Chicken, % (SE) | 10.4 (1.6) | 18.2 (2.3) | 13.9 (1.4) | 0.005 |
| Socio-economic variables | ||||
| Wealth index, % (SE) | 0.414 | |||
| Poor | 47.8 (2.7) | 53.1 (2.9) | 50.2 (2) | |
| Middle | 28.5 (2.4) | 26 (2.6) | 27.4 (1.8) | |
| Rich | 23.6 (2.3) | 20.9 (2.4) | 22.4 (1.7) | |
| Mother education, % (SE) | 0.000 | |||
| No education | 10.7 (1.7) | 47.9 (2.9) | 27.7 (1.8) | |
| Primary | 53.6 (2.7) | 36 (2.8) | 45.5 (2.0) | |
| Secondary + | 35.7 (2.6) | 16.1 (2.2) | 26.8 (1.8) | |
| Wash variables | ||||
| Improved sanitation facilities, % (SE) | 36.0 (2.6) | 31.2 (2.7) | 33.8 (1.9) | 0.196 |
| Improved source of drinking water, % (SE) | 64.0 (2.6) | 46.6 (2.9) | 56.0 (2.0) | 0.000 |
| Child usually defecates in a safe place, % (SE) | 43.2 (2.7) | 26.4 (2.6) | 35.5 (1.9) | 0.000 |
| Anthropometric sample | ||||
| N | 302 | 291 | 593 | |
| HAZ, mean (SD) | −1.38 (±1.04) | −1.74 (±1.06) | −1.56 (±1.06) | <0.001 |
| Stunting, % (SE) | 26.5 (2.5) | 41.9 (2.9) | 34.1 (1.90) | <0.001 |
| WHZ, mean (SD) | −0.81 (±0.95) | −1.08 (±0.94) | −1.05 (±0.95) | <0.001 |
| MUAC, mean (SD) | 14.07 (±1.05) | 13.74 (±1.11) | 13.91 (±1.09) | <0.001 |
| Wasting: WHZ < −2, % (SE) | 9.9 (1.7) | 12.7 (2.0) | 11.3 (1.3) | 0.285 |
| Wasting ‡: WHZ < −2 and/or MUAC < 12.5, % (SE) | 11.3 (1.8) | 15.8 (2.1) | 13.5 (1.4) | 0.105 |
SD = Standard Deviation. SE = Standard Error. HAZ = Height for Age Z-scores. WHZ = Weight for Height Z-scores; MUA = Middle Upper Arm Circonference; † Testing differences in the distribution of the variable between the two regions: Chi-square test for categorical variable and one-way anova for normally distributed variables; ‡ Following WHO definition, the MUAC cut-off was applied for children who are more than 6 months.
A majority of mothers had primary education only (45.5%) with education of the remaining mothers almost equally distributed across no education and secondary education or more (27.7% and 26.8% respectively). One-third of households reported improved sanitation facilities and children defecating in a safe place. Drinking water was reported to come from an improved source by 56% of households. The prevalence of at least one episode of diarrhea in the last 15 days was 13.6%. 34% Of children (N = 593) were found stunted, 11% wasted (WHZ < −2 Z-score). The proportion of children wasted increased to 13.5% when MUAC inferior to 12.5 cm was included as an additional indicator for wasting.
Demographic characteristics across the two regions were comparable, as well as presence of G. duodenalis infection. Parasites were found in 22.3% of the stool samples from Kratie and in 26.9% of those from Ratanak Kiri. Giardia duodenalis was almost the only parasite found in the stool samples (93% in Kratie and 80% in Ratanak Kiri) whereas the presence of other protozoa was found in less than 3% of the samples of both regions. No helminths were found in the stool samples from Kratie and very few in those from Ratanak Kiri (3.8%). Distribution of wealth index was approximately similar for both regions (47.8% and 53.1% poor in Kratie and Ratanak Kiri respectively and an approximately equal distribution for middle and rich). Livestock ownership differed between regions, with pig ownership more present in Ratanak Kiri (53% versus 28%, p < 0.001) and ownership of cows more prevalent in Kratie (62% versus 13%, p < 0.001). Distribution of mother`s education differed between the 2 regions with more mothers having primary (54% vs. 36%) and secondary education (35% vs. 16%), in Kratie versus Ratanak Kiri. For WASH indicators, the prevalence of improved source of drinking water and the prevalence of children who defecated in a safe place were significantly higher in Kratie compared to Ratanak Kiri (64.0% vs. 46.6% and 43.2% vs. 26.4% respectively, p < 0.001 for both indicators).
The proportion of children who usually played outside the home was similar and high in both regions, with >90% of the children playing outside.
3.2. Associations Giardia Duodenalis Presence and Selected Covariates
Table 2, shows the results of logistic regression used to identify variables associated with the presence of G. duodenalis. Among the three different types of livestock ownership, pig ownership was significantly associated with G. duodenalis infection, with odd-ratios at 1.96 and 2.10 for un-adjusted and adjusted analysis respectively.
Table 2.
A logistic regression model explaining the presence of G. duodenalis in children.
| G. duodenalis | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | % c | Unadjusted a | Adjusted b | |||||
| Odd-Ratio | C.I. | p Value | Odd-Ratio | C.I. | p Value | |||
| Animals owned | ||||||||
| Pig | ||||||||
| No | 389 | 18.2 | 1.00 | - | 1.00 | - | ||
| Yes | 250 | 30.4 | 1.96 | (1.35, 2.84) | <0.001 | 2.10 | (1.33, 3.30) | 0.001 |
| Cow | ||||||||
| No | 384 | 21.1 | 1.00 | - | 1.00 | - | ||
| Yes | 255 | 25.9 | 1.31 | (0.90, 1.90) | 0.16 | 1.15 | (0.71, 1.85) | 0.577 |
| Chicken | ||||||||
| No | 89 | 20.2 | 0.83 | (0.48, 1.44) | 0.502 | 1.08 | (0.59, 2.00) | 0.800 |
| Yes | 550 | 23.4 | 1.00 | - | 1.00 | - | ||
| Socio-economic position | ||||||||
| Wealth index d | ||||||||
| Poor | 321 | 26.8 | 1.00 | - | 1.00 | - | ||
| Middle/Rich | 318 | 19.2 | 0.65 | (0.45, 0.94) | 0.023 | 0.86 | (0.56, 1.30) | 0.467 |
| Mother-education | ||||||||
| No education | 177 | 28.2 | 1.00 | - | 1.00 | - | ||
| Primary | 291 | 24.4 | 0.82 | (0.54, 1.25) | 0.357 | 0.91 | (0.55, 1.51) | 0.725 |
| Secondary | 171 | 15.2 | 0.46 | (0.27, 0.77) | 0.004 | 0.49 | (0.26, 0.92) | 0.027 |
| Wash variable | ||||||||
| Sanitation facilities | ||||||||
| Non improved | 423 | 25.8 | 1.00 | - | 1.00 | - | ||
| Improved | 216 | 17.6 | 0.61 | (0.41, 0.93) | 0.021 | 0.73 | (0.45, 1.19) | 0.202 |
| Source of drinking water | ||||||||
| Non improved | 281 | 25.6 | 1.00 | - | 1.00 | - | ||
| Improved | 358 | 20.9 | 0.77 | (0.53, 1.11) | 0.164 | 1.1 | (0.72, 1.70) | 0.657 |
| Usual place child defecates | ||||||||
| Unsafe | 412 | 25.2 | 1.00 | - | 1.00 | - | ||
| Safe | 227 | 18.9 | 0.69 | (0.46, 1.03) | 0.071 | 1.08 | (0.66, 1.76) | 0.759 |
| Demographic variable | ||||||||
| Age in months e | 639 | - | 4.13 | (2.49–6.87) | <0.001 | 4.72 | (2.77, 8.05) | <0.001 |
| Region | ||||||||
| Kratie | 347 | 23.6 | 1.00 | - | 1.00 | - | ||
| Ratanak Kiri | 292 | 22.2 | 0.93 | (0.64, 1.34) | 0.682 | 0.7 | (0.42, 1.19) | 0.186 |
a Unadjusted: univariate analysis; b Adjusted for all the covariates included in the model; c Prevalence of G. Duodenalis; d Middle and Rich categories were merged together because their associated odd-ratio were similar; e Log transformed variable.
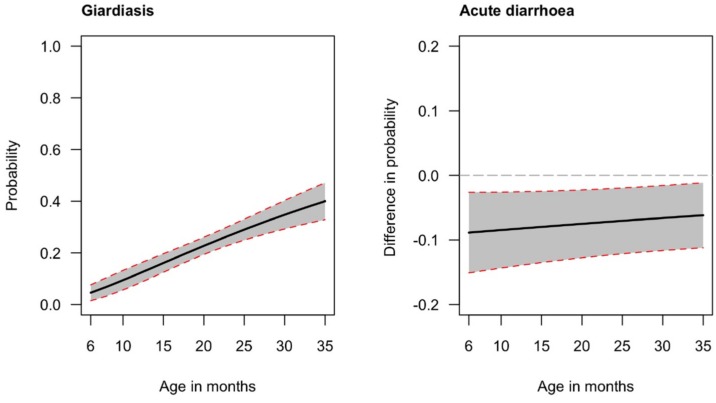
Wash indicators did not show strong associations. Although improved sanitation had a significant protective effect in the univariate analysis, this effect was no longer significant after adjustments (p values 0.021 and 0.202 respectively). Neither the place where the child usually defecated nor the source of drinking water were found associated with the presence of G. duodenalis. In contrast, mother’s education was associated with the presence of the protozoa. Children from a mother with secondary education were significantly less at risk of G. duodenalis presence than those of mothers who had no education, even after adjustment (Odd-Ratio (OR): 0.49; 95% Confident of Interval (CI): 0.26–0.92). Wealth was associated with lower presence of G. duodenalis in the univariate analysis (OR 0.65, p < 0.05), but, not in the adjusted model (p > 0.05). Finally, the age of the child (log transformed in the model) was strongly correlated with the presence of, with higher risk of being infected with higher age. The probability of having G. duodenalis infection increased from an estimated 4% at 6 months of age to 40% at 35 months of age (Figure 1, left panel).
Figure 1.
(Left): Predicted probability of G. duodenalis infection by age. (Right): Average marginal effect of the giardia variable on acute diarrhea. Both, figures were estimated using the multivariate models presented respectively in Table 2 and Table 3. Shaded areas represent 95% CI (Confidence Interval).
3.3. Associations Acute Diarrhea Symptoms and Selected Covariates
Results of the effect of G. duodenalis infection on diarrhea symptoms are shown in Table 3.
Table 3.
Logistic regression model for the association between acute diarrhea and selected covariates.
| Diarrhea | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | %c | Unadjusted a | Adjusted b | |||||
| Odd-Ratio | C.I. | p Value | Odd-Ratio | C.I. | p Value | |||
| Giardia | ||||||||
| No | 492 | 15.0 | 1.00 | - | 1.00 | - | ||
| Yes | 147 | 8.8 | 0.55 | (0.29, 1.02) | 0.058 | 0.45 | (0.23, 0.87) | 0.017 |
| Have Pig | ||||||||
| No | 385 | 8.5 | 1.00 | - | 1.00 | - | ||
| Yes | 254 | 21.6 | 2.34 | (1.45, 3.80) | 0.001 | 2.00 | (1.19, 3.35) | 0.009 |
| Wealth index d | ||||||||
| Poor | 321 | 18.4 | 1.00 | - | 1.00 | - | ||
| Middle/Rich | 318 | 8.8 | 0.43 | (0.27, 0.69) | 0.001 | 0.54 | (0.32, 0.92) | 0.023 |
| Sanitation facilities | ||||||||
| Non improved | 423 | 16.3 | 1.00 | - | 1.00 | - | ||
| Improved | 216 | 8.3 | 0.48 | (0.28, 0.84) | 0.011 | 0.71 | (0.38, 1.33) | 0.286 |
| Usual place child defecates | ||||||||
| Unsafe | 412 | 17.0 | 1.00 | - | 1.00 | - | ||
| Safe | 227 | 7.5 | 0.48 | (0.27, 0.84) | 0.010 | 0.73 | (0.38, 1.39) | 0.34 |
| Age in months | 639 | - | 0.97 | (0.95, 1.00) | 0.056 | 0.98 | (0.95, 1.01) | 0.211 |
| Region | ||||||||
| Kratie | 347 | 7.2 | 1.00 | - | 1.00 | - | ||
| Ratanak Kiri | 292 | 21.2 | 3.42 | (2.12, 5.69) | <0.001 | 2.69 | (1.59, 4.55) | <0.001 |
a Have Pig, sanitation facilities and usual place child defecates were adjusted for region; b Adjusted for all the covariates in the model; c Prevalence of diarrhea; d Middle and rich categories were merged together because their associated odd-ratios were similar.
The covariates selected for the final model were: household owns pigs, wealth index, sanitation facilities, usual place where the child defecates, age and region. Surprisingly, once adjusted, the odd-ratios for G. duodenalis infection showed a significant protective effect on diarrhea (OR 0.45, p < 0.05). This significant effect is shown on the right panel of Figure 1, where the average marginal effect of the G. duodenalis infection on acute diarrhea is plotted for the 6 to 35 months of age period. It shows that on average the probability of a child with G. duodenalis infection having acute diarrhea is 7 percentage points less than for a child without the presence of G. duodenalis cysts. This difference is approximately constant for age, ranging from 8 percentage points at 6 months to 6 percentage points at 35 months. A significant protective effect is also observed for the wealth index variable (OR adjusted: 0.54; 95% CI: 0.32–0.92), indicating that children from richer and middle-class households are less at risk for diarrhea than those from poor families. In contrast, children living in a household that owns pigs are significantly more at risk of diarrhea even after adjustments (OR adjusted 2.00; 95% CI: 0.32–0.92). Improved sanitation facilities show a significant protective effect for diarrhea only in the unadjusted model with the association no longer significant after adjustment.
3.4. Associations Anthropometric Z-Scores and Selected Covariates
Table 4 reports the results of two linear models used to study the relationship between G. duodenalis infection and two anthropometric outcomes: HAZ and WHZ. The final model for WHZ included as non-forced covariates: household owns pigs, mother education and sanitation facilities. The same variables were included in the final model for HAZ, in addition to mother education. The presence of G. duodenalis cysts was found significantly and negatively associated with HAZ only in the unadjusted model (unadjusted coeff: −0.27, p < 0.05).
Table 4.
Linear regression models explaining the associations between anthropometric z-score and selected covariates.
| HAZ † | WHZ ‡ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Unadjusted a | Adjusted b | Unadjusted a | Adjusted b | |||||||||
| Coeff. | C.I | p Value | Coeff. | C.I. | p Value | Coeff. | C.I | p Value | Coeff | C.I. | p Value | ||
| Giardia | |||||||||||||
| No | 464 | - | - | - | - | - | - | - | - | - | - | - | - |
| Yes | 129 | −0.27 | (−0.47, −0.06) | 0.012 | −0.07 | (−0.27, 0.13) | 0.501 | −0.06 | (−0.25, 0.12) | 0.516 | 0.10 | (−0.09, 0.28) | 0.317 |
| Have Pig | |||||||||||||
| No | 364 | - | - | - | - | - | - | - | - | - | - | - | - |
| Yes | 229 | −0.20 | (−0.38, −0.01) | 0.035 | −0.05 | (−0.23, 0.13) | 0.595 | −0.23 | (−0.40, −0.07) | 0.005 | −0.21 | (−0.37, −0.04) | 0.014 |
| Wealth index | |||||||||||||
| Poor | 290 | - | - | - | - | - | - | ||||||
| Middle | 167 | 0.30 | (0.10, 0.50) | 0.003 | 0.18 | (−0.02, 0.38) | 0.071 | ||||||
| Rich | 136 | 0.56 | (0.34, 0.77) | <0.001 | 0.38 | (0.16, 0.6) | <0.001 | ||||||
| Mother Education | |||||||||||||
| No education | 171 | - | - | - | - | - | - | - | - | - | - | - | - |
| Primary | 263 | 0.31 | (0.09, 0.52) | 0.005 | 0.20 | (−0.01, 0.41) | 0.068 | 0.11 | (−0.09, 0.30) | 0.278 | 0.05 | (−0.14, 0.24) | 0.600 |
| Secondary and more | 159 | 0.56 | (0.32, 0.81) | <0.001 | 0.41 | (0.16, 0.66) | <0.001 | 0.30 | (0.08, 0.52) | 0.008 | 0.27 | (0.05, 0.50) | 0.019 |
| Sanitation facilities | |||||||||||||
| Non improved | 389 | - | - | - | - | - | - | - | - | - | - | - | - |
| Improved | 204 | 0.36 | (0.18, 0.53) | <0.001 | 0.20 | (0.02, 0.38) | 0.032 | 0.15 | (−0.01, 0.31) | 0.059 | 0.10 | (−0.06, 0.26) | 0.226 |
| Gender | |||||||||||||
| Female | 301 | - | - | - | - | - | - | - | - | - | - | - | - |
| Male | 292 | −0.13 | (−0.31, 0.04) | 0.126 | −0.15 | (−0.31, 0.01) | 0.067 | 0.00 | (−0.15, 0.15) | 0.995 | 0.01 | (−0.14, 0.16) | 0.912 |
| Region | |||||||||||||
| Female | 302 | - | - | - | - | - | - | - | - | - | - | - | - |
| Male | 291 | -0.35 | (−0.52, −0.18) | <0.001 | −0.18 | (−0.36, 0.00) | 0.050 | −0.28 | (−0.43, −0.12) | <0.001 | −0.14 | (−0.31, 0.02) | 0.093 |
HAZ = Height for Age z-scores. WHZ= Weight for Age z-scores. Wealth index variables was not included in the final model for WHZ; † R2 for adjusted HAZ model: 14.2 %, p-Value of the model: 0.00001; ‡ R2 for adjusted WHZ model: 9.1%, p-Value of the model: 0.00001; a Have Pig, mother education and sanitation facilites were adjusted for region; b Adjusted for all the covariates in the model, Age and Age squared (coefficients not shown).
A logistic model using the binary stunting variable (HAZ < −2) and including the same covariates was also performed and showed the same results. Indeed, G. duodenalis infection was a strong significant risk factors for stunting in the univariate model (OR = 1.67; 95% CI: 1.12 to 2.49), with a prevalence of stunting of 43% among children with G. duodenalis infection and 31% among children not infected. After inclusion of other covariates, the association between G. duodenalis infection and stunting was no longer significant (p > 0.05). Similar findings and trends were observed for pig ownership (unadjusted coeff. −0.20, p < 0.05). In both, the unadjusted and adjusted models, positive significant associations were noted for wealth index, mother education and sanitation facilities.
There were no significant associations between the presence of G. duodenalis cysts and WHZ in the unadjusted nor adjusted models. In contrast, children from households that owned pigs had a significantly lower WHZ in both, adjusted and unadjusted models (p < 0.001). Mother education also showed a positive effect on WHZ, with children whose mothers had secondary education or more showing a significantly higher WHZ than those with a mother with no education (p < 0.001). Finally, improved sanitation facilities did not show a significant effect on WHZ scores in either model.
4. Discussion
This study explored associations between exposure to animal feces, morbidity and anthropometric outcomes in children under 5 years of age, in Cambodia. The overall prevalence of G. duodenalis infection in children was found 23%. This is in line with findings from a recent study in Cambodia that presented a cumulative prevalence of 22% [15]. We believe the prevalence of 23% G. duodenalis infection as found in this study represents an underestimation. The 22% prevalence of G. duodenalis infection obtained in the recent study was calculated using different diagnostic techniques (flotation, PCR and formalin-ether concentration), while using only the formalin-ether concentration technique produced a prevalence of only 9.6% (21 out of 218 children). This study used the latter diagnostic technique. The microscopic-based technique to identify G. duodenalis cysts is easy and cheap, but, it lacks good levels of sensitivity. Moreover, collection of stool samples in this study was limited to one stool per child only, whereas, three consecutive stool samples in separate days are recommended for the detection of enteric parasites by microscopy due the discontinuous excretion of eggs, cysts and oocysts in time. Molecular-based techniques with high levels of sensitivity and specificity exist (for example a multiplex quantitative PCR for the presence and intensity of protozoal infection) [20], yet, their price and labor are prohibitive for most studies done in lower and middle-income countries.
Regardless of these considerations, this study showed that household pig ownership was significantly associated with the presence of cysts of G. duodenalis. Giardia duodenalis is considered a complex species with at least eight distinct assemblages (labelled A to H), but, only assemblages A and B have been detected in both, humans and a wide range of other mammalian hosts. The other assemblages are likely to be host-specific [21]. G. duodenalis infection was found common in pigs, particularly in exploitations characterized by intensive farming practices [22,23,24]. In Cambodia, only one epidemiological study [15] could be found that assessed the prevalence and diversity of intestinal parasitic infections in humans and domestic animals. Surprisingly, no evidence of suine giardiasis was found. Transition of G. duodenalis between animals and humans has been reported for dogs and in a lower extent for calves and potentially pigs [25]. Further research to genotyping G. duodenalis would be useful to determine the most likely source of infection.
We found a protective effect of the presence of G. duodenalis cysts on diarrhea, whereas G. duodenalis infection was also associated with lower HAZ scores in the unadjusted model. To unravel this association, it would be recommended to make molecular experiments such as Polymerase Chain Reaction (PCR) and sequencing. Unfortunately, the use of formalin for feces conservation in this study hindered the amplification of DNA. In addition, feces were recorded according to pig, cow and chicken ownership only. Further studies without formalin use and collecting information on additional animal contacts (e.g., dog feces) would be needed to elucidate the origin of the animal reservoir.
WASH indicators did not show significant associations with G. duodenalis infection prevalence. This may indicate that sanitation facilities, source of drinking water and usual place where the child defecates are not linked to the presence of G. duodenalis which is surprising for a water-borne disease. It may also indicate that indicators do not adequately reflect the risk of contamination. Low adoption of hygienic measures and consequent in-house contamination of water resources have been acknowledged as particularly risky behaviors in the literature [26].
The increase in G. duodenalis infection prevalence with increasing age is consistent with findings of studies carried out in preschool children in various countries [27]. The increased prevalence with age was reported to be potentially related to a child’s personal autonomy. Whereas children from 1 to 2 years of age are under surveillance most of time, those from 3 to 5 years old start to have more autonomy and are subject to less surveillance which may imply a decrease in the adoption of good hygienic practices. Consequently, older children are likely to be more exposed to risk factors and more often infected than their younger peers [27].
In regard to the association between G. duodenalis infection and anthropometric z-scores, positive significant associations were found for wealth index, mother education and sanitation facilities suggesting that children from a more educated mother, living in a dwelling with improved sanitation or coming from wealthy families tend to have higher HAZ. Similar factors were previously found to improve growth and nutrition status in the literature in general and in Cambodia more specifically [28,29,30]. Still, two recent large studies in Kenia and Bangladesh did not show an impact of improved WASH on anthropometry. This highlights the complexity of interactions between nutritional status, infection and hygiene [31,32].
Both, adjusted and non-adjusted odd-ratios for G. duodenalis infections showed a strong significant protective effect on diarrhea (Table 3). This is in line with the result of a meta-analysis of 17 studies that examined the association between diarrhea and G. duodenalis infection in young children in developing countries [33]. This latter analysis concluded that there was evidence of a significant adverse association between G. duodenalis infection and acute diarrhea. It showed that giardiasis was associated with a 40% lower likelihood of acute diarrhea in children from low-income countries. On the other hand, it showed that G. duodenalis infection is positively associated with persistent diarrhea in these populations. This is different from what is usually observed in developed countries, where children and adults are usually at risk of acute diarrhea when they encounter G. duodenalis infection [34,35,36]. The difference between low and middle-income countries (LMIC) vs. high income countries could be explained by the age of initial exposure and the frequency of re-exposure, as G. duodenalis in LMIC is endemic, leading to an initial infection in the first weeks of life and a rapid acquisition of immunity [37,38,39]. Other possible mechanisms reported to protect against symptomatic G. duodenalis infection in LMIC include the presence of anti- G. duodenalis secretory immunoglobulin A in breast-milk [40,41] and differences in the small intestine [33]. Recent observations have demonstrated that this effect may also be due to a direct immune-modulating effect of the parasite via its cathepsin B cysteine protease which cleaves pro-inflammatory chemokine, more specifically the Interleukin 8 (IL8 or chemokine (C-X-C motif) ligand 8 (CXCL8)) [42]. Robertson et al. (2010) document how new assemblages of G. duodenalis might cause severe symptoms when they first appears in a population. The latter could contribute to explaining the contradictory literature on the relationship between diarrhea and G. duodenalis infection [43]. The immune status of the host and the “strain” of the parasite appear to influence susceptibility to infection, as well as the severity of clinical signs [43]. In addition, G. duodenalis infection may increase the virulence of commensal microbiota bacteria during the acute phase of the infection. In turn, these altered microbiota induce a host inflammatory response, which may be responsible, at least in part, for the long-term, post-infectious, complications seen in giardiasis [42]. Giardia duodenalis infection could protect against diarrhea, for example by competing with or suppressing other enteric pathogens (virus, bacteria or parasite), or by inducing changes in mucosal immunity [43].
In this study, a significant univariate association was noted for the presence of G. duodenalis and HAZ scores, but, this association was not seen after adjustments. The positive association between stunting and G. duodenalis infection is in line with several studies that have shown that G. duodenalis infection may impair linear growth, presumably by reducing intake and/or causing malabsorption of nutrients [10,43,44]. Children with G. duodenalis infection were found to have lower serum zinc and iron status, both acknowledged for their importance in growth [45,46]. The association between livestock and WHZ was found significant, yet, not for HAZ. This is in line with results in the literature that report presence of livestock and improved HAZ because the livestock is a source of food and nutrition, yet, it may also represent a risk from exposure to animal feces harmful to child nutrition status [26].
Our analysis showed that pig ownership represents an increased risk of having acute diarrhea (Table 3). Significant positive associations between livestock and diarrhea illness among children and adults have been documented in the literature. A systematic review of 29 studies examining the association between domestic animal husbandry and diarrheal infections found consistent evidence of a positive association [8]. On the other hand, a recent assessment of Demographic Health Surveys (DHS) from 30 sub-Saharan African countries found an inconsistent relationship between childhood diarrhea and household livestock [47]. Among the countries included, 14 indicated livestock ownership as a risk factor whereas 10 exhibited a protective association.
The present study builds on data from the My Health project, a longitudinal study that was not specifically designed for the current research and data should be considered observational in nature. We acknowledge that this study may be subject to bias. Including a selection bias from mothers who did not submit stool samples of their children reportedly because they did not defecate during the proposed timespan (approximately 24 h). Also, recall bias may have occurred where mothers where asked to report on acute diarrhea episodes in their children over the last two weeks. Finally, although models were adjusted for a series of confounders, it is possible that results are biased by unobserved covariates such as factors related to food consumption.
5. Conclusions
The adverse outcomes of malnutrition on the development of a child are well acknowledged as are the broad variety of contextual factors that may impact on child nutritional status. Improved sanitation and protection from human feces can significantly lower the incidence of diarrhea and environmental enteropathy. However, the impact of excessive exposure to animal feces on child’s health and development is less well documented.
We showed that the presence of livestock and more particularly pigs near the main household dwelling is a risk factor for G. duodenalis infection. Although, presence of G. duodenalis was found a protective factor for acute diarrhea, it was associated with stunting. This finding may be indicative of a subclinical condition like EED. The diagnosis and treatment of G. duodenalis infection is complex in LMIC, such as Cambodia (affordability, feasibility, acceptability) and preventive measures that avoid extensive exposure of young children to animal feces may considerably protect against stunting and improve potential for a healthy development. Programs that focus on animal containment, proper animal waste management, strengthened veterinary control of animal health as well as improved adoption of good hygiene practices, and more particularly frequent hand washing with soap could be most effective in a context similar to Cambodia.
The findings also highlight a need for revised metrics and indicators that do not sufficiently consider the dynamics of environmental transmission of pathogens within households [48]. The results support the integration of cross-sector policy measures that reinforce comprehensive early childhood interventions towards improving nutritional status as part of a wider set of child welfare and development measures.
Acknowledgments
The development of the paper was supported by UNICEF National committees (Korean, DFAT Australia and Canadian). We would like to thank Royal Government of Cambodia for the data collection in every district. We would also like to thank J. van Geystelen for suggestions and editing.
Author Contributions
Y.C., R.H., L.G., A.L., F.T.W., J.B. and E.P. developed the survey design and data collection. Y.C., L.G., A.L., F.T.W. and E.P. conceived and designed the analysis, and analyzed the data. Y.C., R.H., L.G., A.L., F.T.W., J.B. and E.P. contribute to the writing of the manuscript. All co-authors reviewed and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The opinions and statements in this article are those of the authors and may not reflect official policies or opinion of the organizations that they belong.
References
- 1.Hoddinott J., Behrman J., Maluccio J., Melgar P., Quisumbing A., Ramirez-Zea M., Stein A., Yount K., Martorell R. Adult consequences of growth failure in early childhood. Am. J. Clin. Nutr. 2013;98:1170–1178. doi: 10.3945/ajcn.113.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horton S., Alderman H., Rivera J.A. Hunger and Malnutrition, Copenhagen Consensus 2008 Challenge Paper. Copenhagen Consensus Center; Tewksbury, MA, USA: 2008. [Google Scholar]
- 3.UNICEF-WHO . Why Children Are Still Dying, and What Can Be Done? World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- 4.Dangour A., Watson L., Cumming O., Boisson S., Che Y., Velleman Y., Cavill S., Allen E., Uauy R. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst. Rev. 2013;8 doi: 10.1002/14651858.CD009382.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Save-the-Children, Ethiopia. The-Manoff-Group-Inc . Water, Hygiene and Sanitation (WASH) in Rural Households in Amhara, Oromia, SNNP and Tigray. United States Agency for International Development (USAID); Addis Ababa, Ethiopia: 2013. [Google Scholar]
- 6.Taylor L., Latham S., Woolhouse M. Risk factor for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American-Thoracic-Society Respiratory Health Hazards in Agriculture. Am. J. Respir. Crit. Care Med. 1998;158:S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 8.Zambrano L., Levy K., Menezes N., Freeman M. Human diarrhea infections associated with domestic animal husbandry: a systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2014;108:313–325. doi: 10.1093/trstmh/tru056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organisation . The World Health Report, Fighting Diseases, Fostering Development. WHO; Geneva, Switzerland: 1996. [Google Scholar]
- 10.Andrew Thompson R.C. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. Parasites Wildl. 2000;30:1259–1267. doi: 10.1016/S0020-7519(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 11.Mbuya M., Humphrey J. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: an opportunity for stunting reduction in developing countries. Matern. Child. Nutr. 2016;12(Suppl. 1):106–120. doi: 10.1111/mcn.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bless P., Schär F., Khieu V., Kramme S., Odermatt P. High prevalence of large trematode eggs in schoolchildren in Cambodi. Acta Trop. 2015;141:295–302. doi: 10.1016/j.actatropica.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Lin A., Arnold B., Afreen S., Goto R., Huda T., Haque R., Raqib R., Unicomb L., Ahmed T., Colford J.J., et al. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am. J. Trop. Med. Hyg. 2013;89:130–137. doi: 10.4269/ajtmh.12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute of Statistics. Directorate General for Health. ICF International . Cambodia Demographic and Health Survey 2014. National Institute of Statistics; Phnom Penh, Cambodia: 2014. [Google Scholar]
- 15.Schär F., Inpankaew T., Traub R., Khieu V., Dalsgaard A., Chimnoi W., Chhoun C., Sok D., Marti H., Muth S., et al. The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village. Parasitol. Int. 2014;63:597–603. doi: 10.1016/j.parint.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Khieu V., Hattendorf J., Schär F., Marti H., Char M., Muth S., Odermatt P. Strongyloides stercoralis infection and re-infection in a cohort of children in Cambodia. Parasitol. Int. 2014;63:708–712. doi: 10.1016/j.parint.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 17.De Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 18.Filmer D., Prichett L. Estimating wealth effects without expenditure data—Or tears: An application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer D.J., Lemeshow S., Sturdivant R. Applied Logistic Regression. 3rd ed. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2013. Model-Building Strategies and Methods for Logistic Regression. [Google Scholar]
- 20.Campbell S.J., Nery S.V., D’Este C.A., Gray D.J., McCarthy J.S., Traub R.J., Andrews R.M., Llewellyn S., Vallely A.J., Williams G.M., et al. Water, sanitation and hygiene related risk factors for soil transmitted helminth and Giardia duodenalis in rural communities in Timor-Leste. Int. J. Parasitol. 2016;46:771–779. doi: 10.1016/j.ijpara.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Faria C., Zanini G., Dias G., da Silva S., do Céu Sousa M. New multilocus genotype of Giardia lamblia human isolates. Infect. Genet. Evol. 2017;54:128–137. doi: 10.1016/j.meegid.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Armson A., Yang R., Thompson J., Johnson J., Reid S., Ryan U. Giardia genotypes in pigs in Western Australia: prevalence and association with diarrhea. Exp. Parasitol. 2009;121:381–383. doi: 10.1016/j.exppara.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Hamnes I., Gjerde B., Forberg T., Robertson L. Occurrence of Cryptosporidium and Giardia in suckling piglets in Norway. Vet. Parasitol. 2007;144:222–233. doi: 10.1016/j.vetpar.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y., Xiao L. Zoonotic Potential and Molecular Epidemiology of Giardia Species and Giardiasis. Clin. Microbiol. Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farzan A., Parrington L., Coklin T., Cook A., Pintar K., Pollari F., Friendship R., Farber J., Dixon B. Detection and Characterization of Giardia duodenalis and Cryptosporidium spp. on Swine Farms in Ontario, Canada. Foodborne Pathog. Dis. 2011;8:1207–1213. doi: 10.1089/fpd.2011.0907. [DOI] [PubMed] [Google Scholar]
- 26.Heady D., Nguyen P., Kim S., Rawat R., Ruel M., Menon P. Is exposure to animal feces harmful to child nutrition and health outcomes? A multicountry observationnal analysis. Am. J. Trop. Med. Hyg. 2017;96:961–969. doi: 10.4269/ajtmh.16-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Antoli C., Gozalbo M., Pavon A., Escobedo P., Toledo R., Esteban J.G. Enteroparasites in preschool children on the pacific region of Nicaragua. Am. J. Trop. Med. Hyg. 2017;98:1–6. doi: 10.4269/ajtmh.17-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda N., Irieb Y., Shibuyac K. Determinants of reduced child stunting in Cambodia: analysis of pooled data from three Demographic and Health Surveys. Bull. World Health Organ. 2013;91:341–349. doi: 10.2471/BLT.12.113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greffeuille V., Sophonneary P., Laillou A., Gauthier L., Hong R., Hong R., Poirot E., Dijkhuizen M., Wieringa F., Berger J. Persistent Inequalities in Child Undernutrition in Cambodia from 2000 until Today. Nutrients. 2017;8:297. doi: 10.3390/nu8050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chhoun P., Pal K., Oy S., Collins C., Tout S., Yi S. Social Determinants of Maternal and Child Undernutrition in Cambodia: A Systematic Review. Int. J. Food. Sci. Nutr. 2016;3:331–337. doi: 10.15436/2377-0619.16.881. [DOI] [Google Scholar]
- 31.Null C., Stewart C., Pickering A., Dentz H., Arnold B., Arnold C., Benjamin-Chung J., Clasen T., Dewey K., Fernald L., et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob. Health. 2018;6:316–329. doi: 10.1016/S2214-109X(18)30005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luby S., Rahman M., Arnold B., Unicomb L., Ashraf S., Winch P., Stewart C., Begum F., Hussain F., Benjamin-Chung J., et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: A cluster randomised controlled trial. Lancet Glob. Health. 2018;6:302–315. doi: 10.1016/S2214-109X(17)30490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muhsen K., Levine M. A Systematic Review and Meta-analysis of the Association between Giardia lamblia and Endemic Pediatric Diarrhea in Developing Countries. Clin. Infect. Dis. 2012;55(Suppl. 4):S271–S293. doi: 10.1093/cid/cis762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz D., Heisey-Grove D., Beach M., Dicker R., Matyas B. Porlonged outbreak of giardiasis with two modes of transmission. Epidemiol. Infect. 2006;134:935–941. doi: 10.1017/S0950268805005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nash T., Herrington D., Losonsky G., Levine M. Experimental human infections with Giardia lamblia. J. Infect. Dis. 1987;156:974–984. doi: 10.1093/infdis/156.6.974. [DOI] [PubMed] [Google Scholar]
- 36.Nygård K., Schimmer B., Søbstad Ø., Walde A., Tveit I., Langeland N., Hausken T., Aavitsland P. A large community outbreak of waterborne giardiasis-delayed detection in a non-endemic urban area. BMC Public Health. 2006;6:141. doi: 10.1186/1471-2458-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollm-Delgado M., Gilman R., Bern C., Cabrera L., Sterling C., Black R., Checkley W. Lack of an adverse effect of Giardia intestinalis infection on the health of Peruvian children. Am. J. Epidemiol. 2008;168:647–655. doi: 10.1093/aje/kwn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasan K., Pathela P., Alam K., Podder G., Faruque S., Roy E., Fazlul Haque A., Haque R., Albert M., Siddique A., et al. Aetiology of diarrhoea in a birth cohort of children aged 0–2 Year(s) in rural mirzapur, Bangladesh. J. Health Popul. Nutr. 2006;24:25–35. [PubMed] [Google Scholar]
- 39.Valentiner-Branth P., Steinsland H., Fischer T., Perch M., Scheutz F., Dias F., Aaby P., Mølbak K., Sommerfelt H. Cohort Study of Guinean Children: Incidence, Pathogenicity, Conferred Protection, and Attributable Risk for Enteropathogens during the First 2 Years of Life. J. Clin. Microbiol. 2003;41:4238–4245. doi: 10.1128/JCM.41.9.4238-4245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walterspiel J., Morrow A., Guerrero M., Ruiz-Palacio G., Pickering L. Secretory anti-Giardia lamblia antibodies in human milk: protective effect against diarrhea. Pediatrics. 1994;93:28–31. [PubMed] [Google Scholar]
- 41.Mahmud M., Chappel C., Hossain M., Huang D., Habib M., DuPont H. Impasct on breast-feeding on Giardia lamblia infections in Bilbeis, Egypt. Am. J. Trop. Med. Hyg. 2001;65:257–260. doi: 10.4269/ajtmh.2001.65.257. [DOI] [PubMed] [Google Scholar]
- 42.Buret A., Amat C., Manko A., Beatty J., Hallize M., Bhargava A.M.J., Cotton J. Giardia duodenalis: New research developments in pathophysiology, pathogenesis, and virulence factors. Curr. Trop. Med. Rep. 2015;2:110–118. doi: 10.1007/s40475-015-0049-8. [DOI] [Google Scholar]
- 43.Veenemans J., Mank T., Ottenhof M., Baidjoe A., Mbugi E., Demir A., Wielders J., Savelkoul H., Verhoef H. Protection against diarrhoea associated with Giradia intestinalis is lost with multi-nutrient supplementation: a study in Tanzanian children. PLoS Negl. Trop. Dis. 2011:1–10. doi: 10.1371/journal.pntd.0001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosek M.N. MAL-ED Network Investigators. Causal pathways from enteropathogens to environmental enteropathy: Findings from the MAL-ED Birth cohort study. EBioMedicine. 2017;18:109–117. doi: 10.1016/j.ebiom.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson L., Hanevik K., Escobedo A., Morch K., Langeland N. Giardiasis—Why do the symptoms sometimes never stop? Trends Parasitol. 2010;26:75–82. doi: 10.1016/j.pt.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Zarebavani M., Dargahi D., Einollahi N., Dashti N., Mohebali M., Rezaeian M. Serum levels of zinc, copper, vitamin B12, folate and immunoglobulins in individuals with giardiasis. Iran J. Public Health. 2012;41:47–53. [PMC free article] [PubMed] [Google Scholar]
- 47.Kaur M., Graham J., Eisenberg J. Livestock Ownership Among Rural Households and Child Morbidity and Mortality: An Analysis of Demographic Health Survey Data from 30 Sub-Saharan African Countries (2005–2015) Am. J. Trop. Med. Hyg. 2017;96:741–748. doi: 10.4269/ajtmh.16-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair R.G., Gerba C.P. Microbial contamination in kitchens and bathrooms of rural Cambodian village households. J. Appl. Microbiol. 2011;52:144–149. doi: 10.1111/j.1472-765X.2010.02978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]