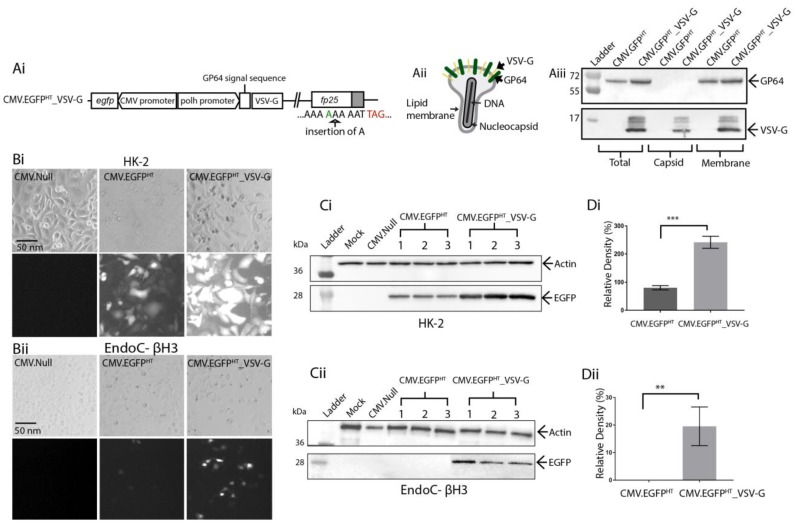
Figure 4.
(A) schematic representation of the VSV-G pseudotyped BacMam vector construct. (Ai) 21-amino-acid ectodomain of VSV-G, with its transmembrane and cytoplasmic tail domains (aa 442–511) [29] was inserted into the HT virus genome under the control of the polh promoter as a fusion to the gp64 signal peptide. TAG, stop codon (red) (Aii) Schematic representation of the BV particle with VSV-G incorporation in the envelope surface alongside the native GP64 protein. (Aiii) immunoblot analysis of intact and fractionated BV (nucleocapsid and membrane envelope) were analysed by Western blotting using anti-VSV-G or anti-GP64 specific antibodies. Molecular weight markers are in kDa. (B) bright field and fluorescence images of (Bi) HK-2 (MOI 150) and (Bii) EndoC-βH3 (MOI 250) cells transduced with CMV.EGFPHT VSV-G pseudotyped or non-pseudotyped BacMam vectors. Images were taken at 72 hpt at 10× using a Zeiss Axiovert 135 inverted epifluorescence microscope. Scale bar, 50 nm. (C) immunoblot analysis of transduced (Ci) HK-2 and (Cii) EndoC-βH3 cell lysates harvested at 72 hpt, using target-specific antibodies. Β-actin was used as a loading control. Molecular weight markers in kDa; (D) a bar graph was constructed using GraphPad Prism showing the relative band intensities for EGFP in (Di) HK-2 and (Dii) EndoC-βH3 cells. Each sample was normalised against β-actin. Error bars represent ± SD (n = 3) and indicate a significant difference between CMV.EGFPHT and CMV.EGFPHT_VSV-G transduced cells as analysed using a Student’s t-test (p < 0.05).