Figure 1.
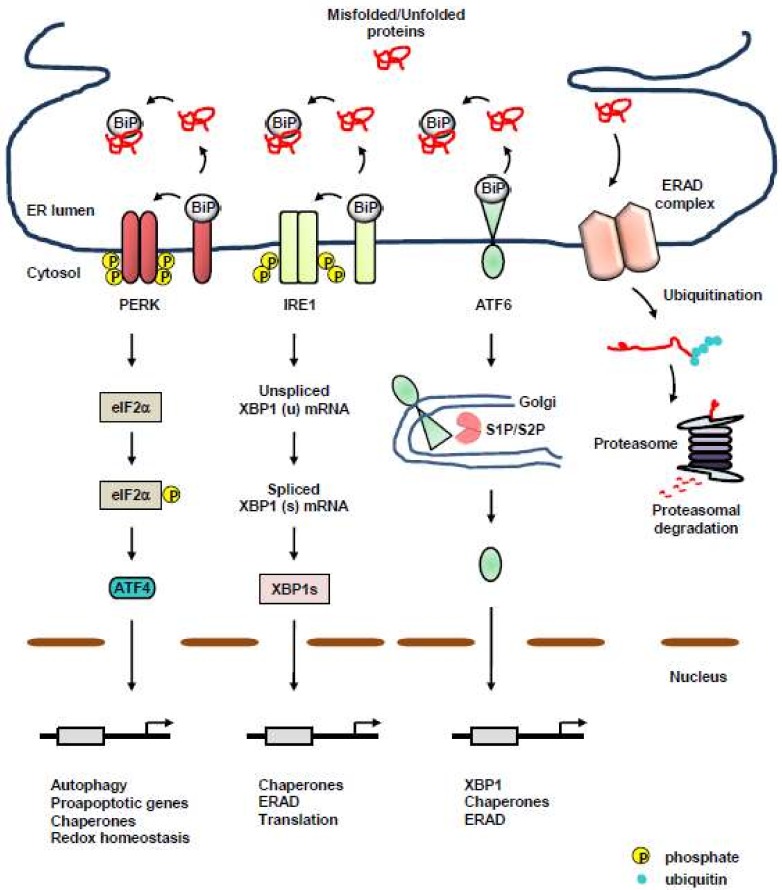
Protein quality-control machineries of the endoplasmic reticulum (ER). Protein quality-control machineries of the ER consist of three axes: acceleration of adequate protein folding, activation of the unfolded protein response (UPR), and protein clearance via ER-associated degradation (ERAD), or autophagy. UPR is composed of three transmembrane ER-resident stress sensors, inositol-requiring protein 1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase RNA (PKR)-like ER kinase (PERK). Under unstressed conditions, the luminal domains of these UPR sensors are kept inactive via binding to a chaperone, binding immunoglobulin protein (BiP). Upon ER stress, BiP dissociates from the ER sensors, leading to the activation of UPR. The PERK/ATF4 axis induces the expression of chaperones and genes involved in autophagy, apoptosis, and redox homeostasis. The IRE1/X-box binding protein 1 (XBP1) axis facilitates the transcription of a subset of UPR genes linked to adequate folding and secretion of proteins, as well as ERAD [23,24,25,26]. Activated ATF6 induces the expression of chaperones, XBP1, and genes involved in ERAD. ERAD is also the protein quality-control machinery of the ER for removing terminally misfolded, unassembled, or tightly regulated proteins via the cytosolic ubiquitin–proteasome system (UPS). Following retrotranslocation across the ER membrane, ERAD substrates are ubiquitinated and degraded by the proteasome in the cytoplasm. Black arrow, facilitation; red line, misfolded/unfolded protein.