Abstract
Purpose
NKAP plays an important role in transcriptional repression, T-cell development, maturation and function acquisition, maintenance and survival of hematopoietic stem cells, and RNA splicing. In this study, we tried to explore the physiological role of NKAP in breast cancer.
Methods
We investigated NKAP expression in breast cancer patients and normal controls and its correlation with survival in breast cancer patients by searching on GEPIA. We knocked down the expression of NKAP in MCF-7 cells by RNAi technique and studied its effect on cell proliferation, migration, invasion, and apoptosis. And we revealed the effect of NKAP on MCF-7 cells under hypoxic conditions in vitro.
Results
NKAP was differentially expressed in breast cancer and normal tissues and is a potential prognostic indicator of breast cancer. Subsequently, NKAP knockdown significantly inhibited the proliferation and clonality of MCF-7 cells and induced its apoptosis through caspase 3-dependent pathway. In addition, knockdown of NKAP could strongly inhibit the migration and invasion of MCF-7 cells. In MCF-7 cells, NKAP affected the AKT/mTOR signaling pathway and markedly reduced the phosphorylation of AKT and mTOR, as well as the downstream protein. What’s interesting is CoCl2 was found to induce NKAP expression in MCF-7 cells. Downregulation of NKAP hindered the impact of CoCl2 on the MCF-7 cells, including cell proliferation and invasion, by adjusting AKT/mTOR signaling.
Conclusion
NKAP functioned as an oncogene, and its expression was induced by hypoxia in breast cancer via AKT/mTOR signaling pathway.
Keywords: NKAP, breast cancer, hypoxia, AKT/mTOR signaling pathway
Introduction
NKAP is a 47 kD, highly conserved nuclear protein with up to 86% homology in humans and mice, which is first recognized to involve in NF-kB activation in 293 cells.1 It is better known as a negative regulator and transcriptional repressor of Notch signaling by virtue of its ability to control T-cell maturation and gain of work, thus deletion of NKAP results in enhanced expression of Notch targeted genes.2 Subsequent studies have shown that perinatal death and loss of hematopoiesis occur in conditional knockout mice that delete NKAP in the hematopoietic stem cells (HSCs) and all hematopoietic lineages during embryonic development, such as multidirectional defects in the development of lymphocytes, granulocytes, erythrocytes, and megakaryocytes.3 Recent research found that NKAP can interact with a variety of RNA-binding proteins, including RNA helicases, splicing factors, and FUS/TLS.4 The X-inactive-specific transcript regulatory RNA, an epigenetic inactivator of the X chromosome, has also been found to be associated with NKAP.4 In summary, NKAP plays an important role in transcriptional repression, T-cell development, maturation and function acquisition, maintenance and survival of HSCs and RNA splicing. However, we believe that NKAP still has other important features that have not been dredged, especially in the area of cancer. To the best of our knowledge, for the first time, we observed a link between NKAP and breast cancer.
Breast cancer is a major public health problem and the most common cancer among women, accounting for about 16% in the world.5 In clinical treatment, tumor metastasis accounts for 90% of breast cancer deaths. In recent years, there is increasing evidence that oxygen content in tumor tissue is an important determinant of breast cancer metastasis. Ongoing tumor hypoxia leads to more malignant phenotypes. Multiple factors associated with hypoxia contribute to the development of malignancies.
Here, we investigated NKAP expression in breast cancer patients and normal controls and its correlation with survival in breast cancer patients. In order to explore the physiological role of NKAP in breast cancer, we knocked down the expression of NKAP in MCF-7 human breast cancer cells by RNAi technique and studied its effect on cell proliferation, migration, invasion, and apoptosis. And we revealed the effect of NKAP on MCF-7 cells treated by CoCl2 in vitro.
Materials and methods
Materials
DMEM was provided by HyClone Company (Thermo Fisher Scientific, Waltham, MA, USA). FBS was purchased from Thermo Fisher Scientific. Penicillin-streptomycin, 0.25% trypsin, and cell counting kit-8 (CCK-8) agent were purchased from Beijing Solarbio Science & Technology Company (Beijing, China). siRNA for NKAP was purchased from Gene (Shanghai, China). Lipofectamine 2000 was obtained from Thermo Fisher Scientific. Trizol reagent was provided by Thermo Fisher Scientific. RevertAid First Strand cDNA Synthesis Kit and SYBR Premix Ex Taq II were purchased from Takara (Japan). Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit was ordered from Thermo Fisher Scientific. CoCl2 was ordered from Sigma-Aldrich Co. (St Louis, MO, USA). RIPA Lysis Buffer, BCA Protein Assay Kit, and Protease Inhibitor Cocktail were all purchased from CwBio (Beijing, China). Primers were synthesized by Genewiz Company (Beijing, China). Transwell cell culture plates were ordered from EMD Millipore (Billerica, MA, USA). Matrigel Matrix was provided by BD Biosciences (San Jose, CA, USA). Protein Marker was obtained from Thermo Fisher Scientific. Enhanced chemiluminescent (ECL) developer was obtained from PTG (Chicago, IL, USA). This article does not contain any studies with human participants or animals performed by any of the authors; hence ethical approval is not required.
Cell culture and transfection
MCF-7 human breast cancer cells were obtained from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were maintained in DMEM added with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin at 37°C in a humidified atmosphere with 5% CO2 (20% O2, 75% N2). MCF-7 cells were digested when entered in logarithmic phase and then planted into a six-well plate. The fluid was changed in every 3 days. siRNA for NKAP (siNKAP) was transfected into MCF-7 cells using Lipofectamine2000 following the manufacturer’s instructions. A scrambled RNA (siNC) was used as control. RNA was extracted after transfection 24 hours, and protein was dissolved after transfection for 48 hours. Cells were cultured with 50, 100, 150, and 200 µM CoCl2 for different periods of time. MCF-7 cells were incubated with the aforementioned CoCl2 concentrations for 24 hours, and the expression of special gene was measured.
Quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed to confirm the mRNA expression of genes. Total RNA was extracted from cells using Trizol reagent, and then reverse transcribed to cDNAs using the RevertAid First Strand cDNA Synthesis Kit. qRT-PCR was run on an iCycler (Bio-Rad Laboratories Inc., Hercules, CA, USA) using validated primers and SYBR Premix Ex Taq II for detection. The relative quantification was determined using the 2−ΔΔCt method after normalization to the GAPDH level in each sample. Primers used are as follows: NKAP sense: 5′-CGGCAGAAGAGATTAAGTGAG-3′, antisense: 5′-CTCCACTGGTGTATGTTCATC-3′; GAPDH sense: 5′-GCACCGTCAAGGCTGAGAAC-3′, antisense: 5′-TGGTGAAGACGCCAGTGG-3′; HIF 1α sense: 5′-TCTGGGTTGAAACTCAAGCAACTG-3′, antisense: 5′-CAACCGGTTTAAGGACACATTCTG-3′; CXCR4 sense: 5′-TCTGTGACCGCTTCTACC-3′, anti-sense: 5′–AGGATGAGGATGACTGTGG-3′; VEGF sense: 5′-TGCTTCTGAGTTGCCCAGGA-3′, antisense: 5′-TGGTTTCAATGGTGTGAGGACATAG-3′.
Western blot and antibodies
Cells were lysed with ice-cold RIPA buffer and protease inhibitors, and the protein level of cell extracts was analyzed using Western blot assay. Protein concentration was measured using the Bio-Rad protein assay kit. Total proteins (15 µg) were separated by SDS-PAGE and transferred to a PVDF membrane. Then, the membrane was preblocked in TBS containing 5% fat-free milk and 0.1% Tween-20 for 1 hour, and incubated overnight at 4°C with primary antibodies (1:1,000), followed by the secondary antibodies (1:2,000) for 1 hour at room temperature. ECL substrate reagent was used to generate chemiluminescent signals. The relative expression levels of proteins were normalized against GAPDH and quantified with QUANTITY ONE software. Western blotting was performed three times. Antibodies used are as follows: rabbit anti-human NKAP, Bax, caspase 3, caspase 9, mTOR, p-mTOR, p70, and HIF-1 were obtained from Cell Signaling Technology (Danvers, MA, USA); mouse anti-human Bcl, AKT, p-AKT, Cyclin D1, VEGF, CXCR4, and GAPDH were obtained from Cell Signaling Technology.
CCK-8 assay
CCK-8 assay was performed to confirm the proliferation of cells following the manufacturer’s instructions. About 5×103 cells that transfected siNKAP or siNC were seeded into each well of 96-well plate and cultured overnight. At 24, 48, 72, and 96 hours after transfection, the culture medium was removed, and then fresh DMEM containing 10 µL CCK-8 solution was added and incubated for 2 hours at 37°C. The proliferation of cells was determined by measuring OD value at 450 nm. CCK-8 assay was performed in triplicate.
Clone formation assay
After transfection for 24 hours, 500 cells were planted into a 6 cm dish with 5 mL medium. Cell culture mediums were changed every 3 days. Sustained culturing was performed until the clones could be visible, after that medium was removed and the cells were fixed for 30–60 minutes with 4% paraformaldehyde solution. Following this, the clones were stained with 0.1% crystal violet and were photographed and counted.
Transwell assays
Boyden chambers (8 µm pore size) were performed to confirm the invasion and migration of MCF-7 cells. Matrigel Matrix was used for invasion assay, and uncoated filters were used for migration assay. Cells (1×105) that transfected with siNKAP or siNC were seeded in the upper chamber. Then 600 µL of medium with 10% FBS was added to the lower chamber to guide cell migration and invasion. After incubation for 48 hours, the residual cells on the upper chamber were removed by scraping and the cells that migrated and adhered onto the lower chamber were fixed by 4% paraformaldehyde for 30 minutes, then stained with 0.1% crystal violet for 20 minutes. Migrated cells were quantified by counting in five separated visual fields. Each migration and invasion assay were repeated in three independent experiments.
Flow cytometry for apoptosis detection
Cell apoptosis was analyzed using Annexin V-FITC Apoptosis Detection Kit I. After transfection for 48 hours, MCF-7 cells were harvested and washed with precooled PBS. Then the cells were centrifuged, and the supernatant was carefully removed. Annexin V binding buffer was added to resuspend cells to 1–5×106/mL. About 100 µL of cell suspension was incubated with 5 µL of Annexin V/FITC mix for 5 minutes. Then 10 µL of PI dye and 400 µL of PBS was added before detecting apoptosis by flow cytometry. Statistical analysis was performed using Flowjo software.
Statistical analysis
In this study, all experiments were performed more than three times. SPSS 20.0 statistical analysis software was used to analyze all experimental data. The comparison between two groups was performed by Student’s t-test, one-factor ANOVA test was used to compare the values obtained in three or more groups, followed by Tukey’s post hoc test, and P<0.05 was considered statistically significant. Results were expressed as means ± SD.
Results
High NKAP expression in human breast cancer represented a poor prognosis
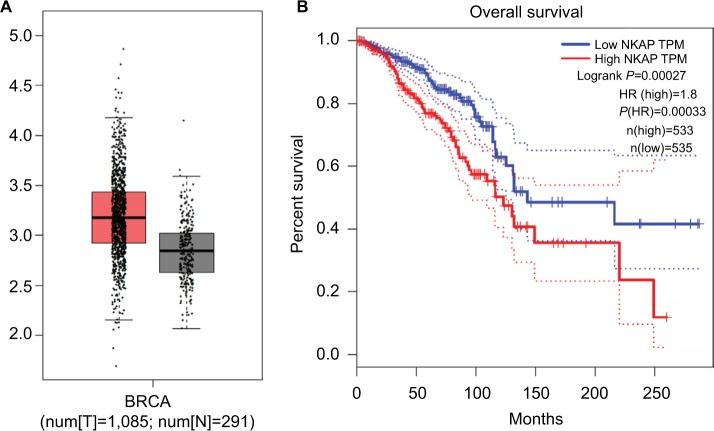
The TCGA database is a gene information database, which contains information of 1,085 patients with breast cancer (breast invasive carcinoma, BRCA) and 291 normal individuals. GEPIA is an online visualization site based on TCGA and GTEx data, enabling various online analysis including differential expression analysis, box plotting, correlation analysis, patient survival analysis, and so on. The box plots were shown in Figure 1A. It revealed that NKAP expression was significantly higher in BRCA (P<0.05). The survival curves of BRCA patients with high or low NKAP expression were shown in Figure 1B. The result displayed that the survival rate of patients with a high NKAP expression was markedly lower in comparison with those with a low NKAP expression. It showed that high NKAP expression represented a poor prognosis, and NKAP might play a role in promoting breast cancer progression.
Figure 1.
The boxplot of the mRNA expression of NKAP in BRCA and survival percentage of BRCA patients with high or low NKAP expression.
Notes: High NKAP expression in breast cancer represented a poor prognosis. (A) The boxplot of mRNA expression of NKAP in BRCA. The red and gray boxes represent breast tumor and normal tissues, respectively. The data were obtained from TCGA database. The y-axis indicates the log2-transformed gene expression level. (B) The survival percentage of BRCA patients with high or low NKAP expression.
Abbreviations: BRCA, breast invasive carcinoma; N, normal; T, tumor; TPM, trans per million.
Downregulation of NKAP inhibited the proliferation and motility of MCF-7 cells
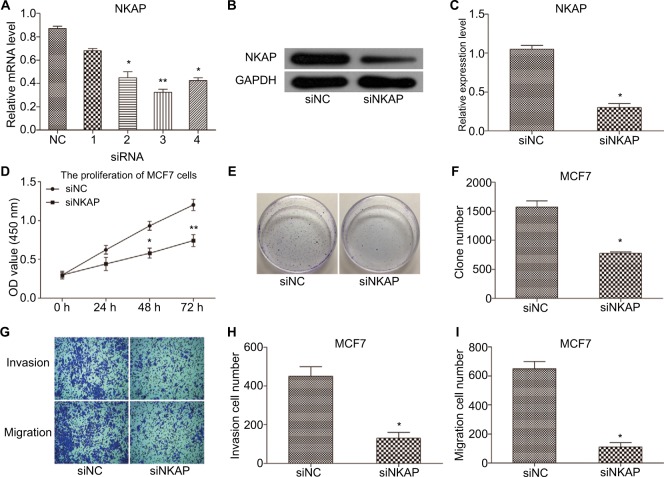
We first tried to understand whether NKAP is involved in the biological behavior regulation of breast cancer cells. We designed four siRNAs to downregulate NKAP expression in MCF-7 cells. As shown in Figure 2A, siRNA3 exhibited the most powerful activity in knocking down NKAP expression and was selected for the follow-up experiments. Western blot results in Figure 2B and C validated the impact of siNKAP on downregulation of NKAP expression.
Figure 2.
Downregulation of NKAP inhibited cell proliferation and migration in MCF-7 cells.
Notes: (A) qRT-PCR demonstrating siRNA 2, 3, and 4 inhibited NKAP expression efficiently. (B and C) Western blot verified the inhibition of NKAP expression. (D) Cell proliferation detected by CCK-8 assay. (E and F) Cell proliferation detected by clone formation assay. (G–I) Cell migration and invasion detected by transwell assay. *P<0.05, **P<0.01 compared with NC.
Abbreviation: NC, negative control.
Unlimited proliferation is the most essential feature of cancer cells, so we examined the effect of NKAP downregulation on MCF-7 cell proliferation by using CCK-8 assay and clone formation assay. As shown in Figure 2D, the OD value of siNKAP transfected cells was significantly decreased compared to that of siNC transfected cells (P<0.05). Clone formation assay also showed the same result (Figure 2E and F). The average clone number was decreased from 1,576±106 of siNC group to 775±32 of siNKAP group (P<0.05).
The effect of NKAP downregulation on MCF-7 cell migration and invasion was also detected using transwell assay. The result shown in Figure 2G, suggested that the migrated cells in siNKAP transfected group were significantly decreased compared to those in siNC group, as well as invaded cells (P<0.05). Quantified results were shown in Figure 2H and I. In summary, siNKAP transfection significantly inhibited the proliferation, migration, and invasion of MCF-7 cells, which implied that NKAP might play an oncogenic role in breast cancer.
Downregulation of NKAP induced apoptosis of MCF-7 cells
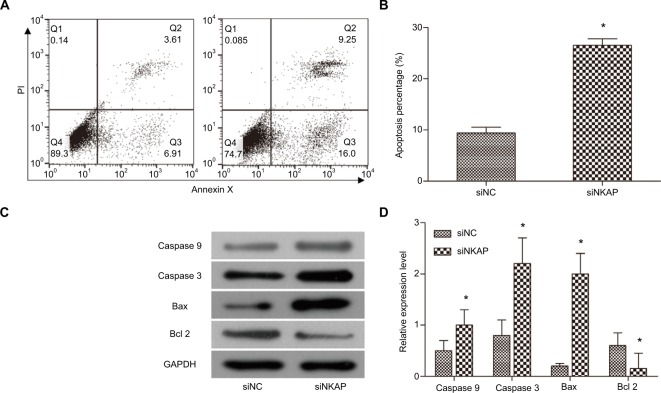
We performed flow cytometry to investigate the role of NKAP on apoptosis of MCF-7 cells. Cell apoptosis analysis (Figure 3A and B) found that siNKAP transfection significantly increased cell apoptosis percentage in MCF-7 cells, from 10.52% of siNC group to 25.25% of siNKAP group (P<0.05). We also detected the expression of apoptosis-associated proteins by Western blot. As shown in Figure 3C and D, siNKAP transfection increased the expression of pro-apoptotic protein caspase 9, active-caspase 3, Bax, and decreased the expression of anti-apoptotic protein Bcl 2. The aforementioned results revealed downregulation of NKAP induced MCF-7 apoptosis.
Figure 3.
Downregulation of NKAP induced apoptosis.
Notes: (A and B) MCF-7 cell apoptosis detected by flow cytometer. (C and D) The expression of apoptosis-associated proteins detected by Western blot. *P<0.05 compared with NC.
Abbreviation: PI, propidium iodide.
Downregulation of NKAP inhibited AKT/mTOR signaling pathway
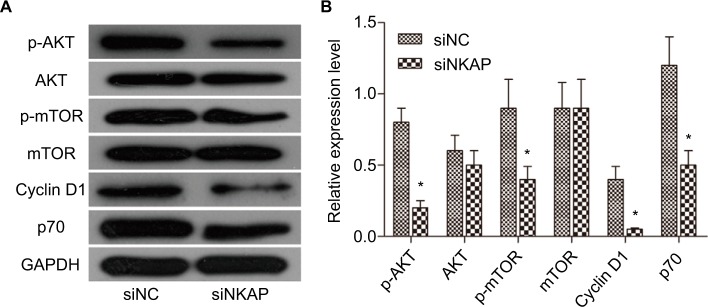
AKT/mTOR signaling pathway is a classic cell survival pathway which is correlated to cell proliferation, differentiation, aggressiveness, metastasis, and apoptosis. Many previous studies have reported that AKT/mTOR signaling pathway is activated in various forms of cancers, including breast cancer.6 In our current research (Figure 4A and B), p-AKT and p-mTOR levels were significantly decreased in siNKAP group compared to siNC group using Western blot, while the expression of AKT and mTOR did not change significantly. The Western blot results presented in Figure 4 also showed that expression of Cyclin D1 and p70 were significantly inhibited by siNKAP transfection. These data suggested that AKT/mTOR signaling pathway was significantly inhibited in siNKAP-transfected MCF-7 cells.
Figure 4.
Downregulation of NKAP inhibited AKT/mTOR signaling pathway.
Notes: Western blot image and quantification assay indicated that siNKAP reduced the phosphorylation of AKT and mTOR without affecting protein expression, as well as decreasing expression of the downstream effector. *P<0.05 compared with NC.
CoCl2 induced the expression of NKAP in MCF-7 cells
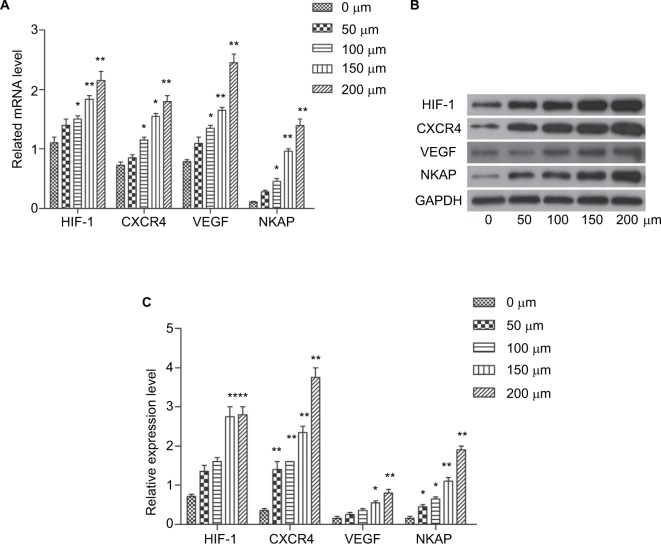
In most solid tumors, including breast cancer tissue, oxygen availability is limited. There is growing evidence that the oxygen content in tumor tissue is a determinant of metastasis. This is an important finding as 90% of breast cancer deaths are caused by metastases. In breast tumors, oxygen concentrations are significantly lower in cells far removed from functional blood vessels compared to normal breast tissue cells, in which cells adapt to hypoxic conditions through the increase of hypoxia inducible factor (HIF) level. Currently, more than 800 HIFs direct target genes have been found, which are involved in cell survival, angiogenesis, metabolic remodeling, immortalization, stem cell maintenance, epithelial–mesenchymal transition, radiation and chemotherapy, invasion, and metastasis. In this study, we found that NKAP may be a new target of hypoxia regulation. As previous studies documented, CoCl2 treatment simulates anoxic environments. Cobalt ions are substrates of iron chelators that can replace the iron ions of the oxygen sensor hemoglobin and bind to high concentrations of oxygen leading to the entry of molecules into the deoxygenation phase.7 In the present study, MCF-7 cells were treated in vitro with different concentrations of CoCl2 (50, 100, 150, and 200 µM) to find the optimal hypoxic model. As shown in Figure 5, the mRNA levels and protein levels of HIF-1, CXCR4, and VEGF were significantly increased by incubation with CoCl2 (P<0.05). The expression of NKAP was also significantly upregulated following CoCl2 dispositions (P<0.05) and 200 µM CoCl2 was used for further experiments. Hypoxic MCF-7 cells (maintained with a gas mixture containing 1% O2, 5% CO2, and 94% N2 for 24 hours) also exhibited the same characteristics (Figure S1A–C). The expression of HIF-1, CXCR4, and VEGF were induced by hypoxia, and NKAP was consistent with their expression trends. This result is so interesting that NKAP may be an oncogene to promote breast cancer progression, and its expression was induced in tumor hypoxia.
Figure 5.
CoCl2 induced the expression of HIF-1, CXCR4, VEGF, and NKAP.
Notes: (A) qRT-PCR demonstrating the mRNA expression of HIF-1, CXCR4, VEGF, and NKAP following treatment with CoCl2. (B and C) Western blot verified the protein expression of HIF-1, CXCR4, VEGF, and NKAP following treatment with CoCl2. *P<0.05, **P<0.01 compared with NC.
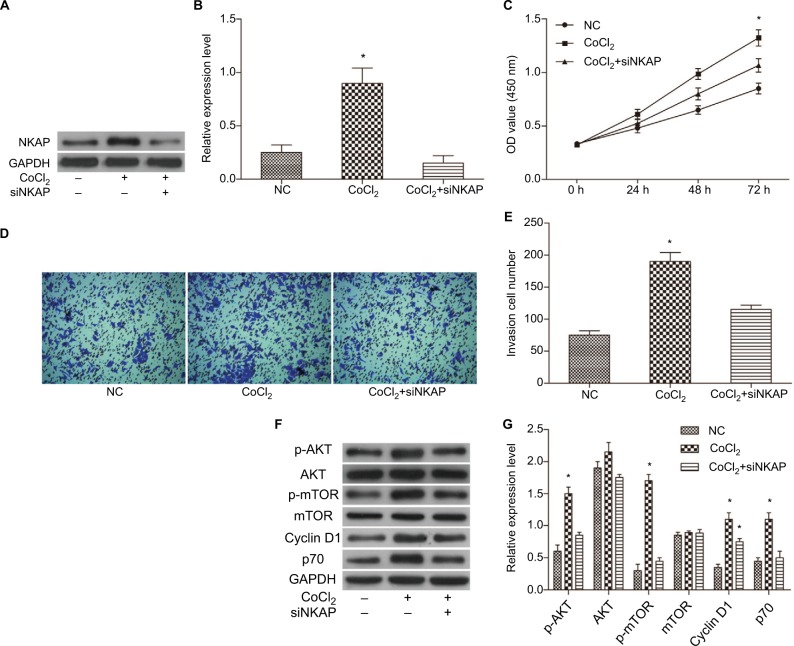
Downregulation of NKAP hindered the impact of CoCl2 on the MCF-7 cells
Western blot results showed that siNKAP could also effectively inhibit the expression of NKAP in MCF-7 cells under CoCl2-treated (Figure 6A and B) and hypoxia (Figure S1A–C) condition. We further detected the proliferation and invasion of MCF-7 cells in negative control group (NC), 200 µM CoCl2-treated hypoxia group (CoCl2), and 200 µM CoCl2-treated plus siNKAP transfection group (CoCl2 + siNKAP), respectively. CCK-8 results (Figure 6C) validated that 200 µM CoCl2 treatment promoted cell proliferation, while siNKAP transfection prevented the promotion of cell proliferation by CoCl2 treatment. Transwell results (Figure 6D and E) suggested that siNKAP transfection also inhibited the promotion of cell migration by CoCl2 treatment. The proliferation and migration of hypoxic MCF-7 cells were also promoted, and this situation was reversed by siNKAP transfection (Figure S1D–F). We still analyzed the activation of the ATK/mTOR signaling pathway. Figure 6F and G depicted the results for CoCl2 treatment promoted the expression of p-AKT, p-mTOR, and downstream target proteins Cyclin D1 and p70 by Western blot. However, these protein levels did not significantly change in CoCl2 + siNKAP compared to NC (Figure 6F and G). Although the expression of Cyclin D1 in CoCl2 + siNKAP was increased compared with NC, it was still downregulated compared with CoCl2, which means that CoCl2 treatment in breast tumor may utilize NKAP to mediate AKT signaling. In hypoxia, the expression of core protein in the AKT/mTOR pathway in MCF-7 cells was consistent with CoCl2 treatment, and knockdown of NKAP inhibited activation of the AKT/mTOR pathway activated by hypoxia (Figure S1G–H). Downregulation of NKAP hindered the impact of CoCl2 on the MCF-7 cells, including cell proliferation and invasion, by adjusting AKT/mTOR signaling.
Figure 6.
Downregulation of NKAP hindered the impact of CoCl2 on the MCF-7 cells.
Notes: (A and B) Western blot verified that siNKAP inhibited NKAP expression efficiently under CoCl2-treated condition. (C) Cell proliferation detected by CCK-8 assay. (D and E) Cell invasion detected by transwell assay. (F and G) Western blot image and quantification assay indicated the activation of AKT/mTOR signaling pathway. *P<0.05 compared with NC.
Abbreviations: CCK-8, cell counting kit-8; NC, negative control.
Discussion
In humans, NKAP activates NF-κB and is a component of the Notch co-repressor complex.8,9 Human NKAP has also been found to bind to different spliceosome complexes, precursor mRNAs, and spliced mRNAs.4,10–13 The role of human NKAP in the regulation of constitutive splicing has been proposed. NKAP is capable of interacting with a variety of RNA binding proteins, including splicing factors. Previous studies have shown that NKAP plays an important role in the development, maturation, and functional acquisition of T cells and iNKT.14 NKAP is also essential for NKT17 cell proliferation and differentiation.14 In the meantime, NKAP is also crucial for the maintenance and survival of HSCs, which may lead to reduced proliferation and increased apoptosis in HSCs.3 NKAP plays an important role in transcriptional repression, T-cell development, maturation, and function acquisition, maintenance, and survival of adult HSCs and RNA splicing. However, people still do not know enough about it and we believe it still has other important roles, especially in the development/prognosis of cancer.
We analyzed the relationship between NKAP and cancer using GEPIA, an online database based on TCGA and GTEx. We found that NKAP was differentially expressed in breast cancer and normal tissues and found that NKAP is a potential prognostic indicator of breast cancer. Subsequently, we knocked down the expression of NKAP in breast cancer cells. The results showed that NKAP knockdown significantly inhibited the proliferation and clonality of MCF-7 cells and induced its apoptosis through caspase-3-dependent pathway. In addition, knockdown of NKAP can significantly inhibit the migration of MCF-7 cells. The above results indicate that NKAP plays a role in promoting the progression of breast cancer. Furthermore, in MCF-7 cells, NKAP affected the AKT/mTOR signaling pathway and significantly reduced the phosphorylation of AKT and mTOR, as well as downstream protein. NKAP in the future is likely to provide a new idea for the treatment of breast cancer. To the best of our knowledge, the present study revealed the association of NKAP with cancer, and it may play an important role in the treatment of other types of cancer.
Hypoxia is one of the important features of tumor microenvironment. Tumor cells will get angiogenesis, migration, and other phenotypes, when it is adapted to a hypoxic environment. It has been suggested that hypoxia inhibits the differentiation of the mammary epithelium and prompts it to maintain stem cell status. The breast cancer stem cell population is thought to participate in the entire process of tumor initiation, drug resistance, and relapse.15 Tumor hypoxia is also closely related to radiotherapy and chemoresistance. Hypoxic tumors respond poorly to radiation therapy, mainly because oxygen is required for the production of reactive oxygen species, and which may further cause DNA damage. In addition, hypoxia reduces the susceptibility of chemotherapeutic agents for the treatment by reducing the susceptibility of DNA damage. Also, hypoxia can modulate tumor response to chemotherapeutic agents by inducing cell cycle arrest and limiting drug delivery.16–18
In this study, we found that the expression of NKAP was induced by hypoxia. Compared with normal cells, cancer cells respond to reduced oxygen availability by enhancing HIF activity.18 Previous studies have shown that HIF-1α can significantly induce and regulate the expression of CXCR4 and its ligand stromal cell derived factor (SDF-1) in breast cancer tissues and cells.19 The CXCR4–SDF-1 interaction potentially mediates the transport of circulating tumor cells in primary breast cancer.19 VEGF is the target gene for HIF-1.20 HIF-1 transcriptional complexes are able to induce the expression of VEGF and induce the corresponding biological effects. VEGF expression is closely related to tumor angiogenesis and lymphangiogenesis in breast cancer.21 We detected the mRNA levels and protein levels of HIF-1, CXCR4, and VEGF by incubation with different concentrations of CoCl2 to establish the optimal anoxic simulation model and what is interesting is CoCl2 was found to induce NKAP expression which is consistent with the expression of HIF-1, CXCR4, and VEGF. Knockdown of NKAP did not affect the expression of HIF1. This suggests that the expression of NKAP is induced by hypoxia, and it may be downstream of HIF1. Downregulation of NKAP hindered the impact of CoCl2 on the MCF-7 cells, including cell proliferation and invasion, by adjusting AKT/mTOR signaling, which means that hypoxia in breast tumor may utilize NKAP to mediate AKT signaling.
Conclusion
In conclusion, we report that NKAP plays an oncogenic function by AKT/mTOR signaling pathway in breast cancer cells, and its expression is induced in tumor hypoxia. Therefore, our work provides a new sight of NKAP in breast cancer progression, which may contribute to the treatment in the future.
Supplementary material
Downregulation of NKAP hindered the impact of hypoxia on the MCF-7 cells.
Notes: (A) qRT-PCR demonstrating the mRNA expression of HIF-1, CXCR4, VEGF, and NKAP in normal (NC), hypoxic (Hypoxia), and hypoxic plus siNKAP transfected (Hypoxia + siNKAP) MCF-7 cells. (B and C) Western blot verified that siNKAP inhibited NKAP expression efficiently under hypoxia condition. (D) Cell proliferation detected by CCK8 assay. (E and F) Cell invasion detected by transwell assay. (G and H) Western blot image and quantification assay indicated the activation of AKT/mTOR signaling pathway. Magnification 100×. *P<0.05 compared with NC.
Acknowledgments
This research was supported by the Natural Science Foundation of Shandong Province (ZR2012HL04) and the Shan-dong Provincial Science and Technology Development Plan (2012YD18062).
Footnotes
Author contributions
All the authors contributed to conception and design, acquisition of data, analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content and provided final approval of the version to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen D, Li Z, Yang Q, Zhang J, Zhai Z, Shu HB. Identification of a nuclear protein that promotes NF-kappaB activation. Biochem Biophys Res Commun. 2003;310(3):720–724. doi: 10.1016/j.bbrc.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 2.Pajerowski AG, Nguyen C, Aghajanian H, et al. NKAP, a novel modulator of Notch signaling, is required for T cell development. Immunity. 2009;30(5):696. doi: 10.1016/j.immuni.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pajerowski AG, Shapiro MJ, Gwin K, et al. Adult hematopoietic stem cells require NKAP for maintenance and survival. Blood. 2010;116(15):2684–2693. doi: 10.1182/blood-2010-02-268391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgute BD, Peche VS, Steckelberg AL, et al. NKAP is a novel RS-related protein that interacts with RNA and RNA binding proteins. Nucleic Acids Res. 2014;42(5):3177–3193. doi: 10.1093/nar/gkt1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Oliveira Andrade F, Fontelles CC, Rosim MP, et al. Exposure to lard-based high-fat diet during fetal and lactation periods modifies breast cancer susceptibility in adulthood in rats. J Nutr Biochem. 2014;25(6):613–622. doi: 10.1016/j.jnutbio.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasari VR, Kaur K, Velpula KK, et al. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS One. 2010;5(4):e10350. doi: 10.1371/journal.pone.0010350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Jiang M, Wang B, Wang C, et al. Inhibition of hypoxia-inducible factor-1alpha and endothelial progenitor cell differentiation by adenoviral transfer of small interfering RNA in vitro. J Vasc Res. 2006;43(6):511–521. doi: 10.1159/000095964. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Li Z, Yang Q, Zhang J, Zhai Z, Shu HB. Identification of a nuclear protein that promotes NF-kappaB activation. Biochem Biophys Res Commun. 2003;310(3):720–724. doi: 10.1016/j.bbrc.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 9.Pajerowski AG, Nguyen C, Aghajanian H, Shapiro MJ, Shapiro VS. NKAP is a transcriptional repressor of notch signaling and is required for T cell development. Immunity. 2009;30(5):696–707. doi: 10.1016/j.immuni.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurica MS, Moore MJ. Capturing splicing complexes to study structure and mechanism. Methods. 2002;28(3):336–345. doi: 10.1016/s1046-2023(02)00240-2. [DOI] [PubMed] [Google Scholar]
- 11.Bessonov S, Anokhina M, Will CL, Urlaub H, Lührmann R. Isolation of an active step I spliceosome and composition of its RNP core. Nature. 2008;452(7189):846–850. doi: 10.1038/nature06842. [DOI] [PubMed] [Google Scholar]
- 12.Ilagan JO, Chalkley RJ, Burlingame AL, Jurica MS. Rearrangements within human spliceosomes captured after exon ligation. RNA. 2013;19(3):400–412. doi: 10.1261/rna.034223.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessonov S, Anokhina M, Krasauskas A, et al. Characterization of purified human Bact spliceosomal complexes reveals compositional and morphological changes during spliceosome activation and first step catalysis. RNA. 2010;16(12):2384–2403. doi: 10.1261/rna.2456210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thapa P, Chen MW, Mcwilliams DC, et al. NKAP Regulates Invariant NKT Cell Proliferation and Differentiation into ROR-γt-Expressing NKT17 Cells. J Immunol. 2016;196(12):4987–4998. doi: 10.4049/jimmunol.1501653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7(10):791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 16.Cho J, Kim D, Lee S, Lee Y. Cobalt chloride-induced estrogen receptor alpha down-regulation involves hypoxia-inducible factor-1alpha in MCF-7 human breast cancer cells. Mol Endocrinol. 2005;19(5):1191–1199. doi: 10.1210/me.2004-0162. [DOI] [PubMed] [Google Scholar]
- 17.Kronblad A, Jirström K, Rydén L, Nordenskjöld B, Landberg G. Hypoxia inducible factor-1alpha is a prognostic marker in premenopausal patients with intermediate to highly differentiated breast cancer but not a predictive marker for tamoxifen response. Int J Cancer. 2006;118(10):2609–2616. doi: 10.1002/ijc.21676. [DOI] [PubMed] [Google Scholar]
- 18.Stoner M, Saville B, Wormke M, Dean D, Burghardt R, Safe S. Hypoxia induces proteasome-dependent degradation of estrogen receptor alpha in ZR-75 breast cancer cells. Mol Endocrinol. 2002;16(10):2231–2242. doi: 10.1210/me.2001-0347. [DOI] [PubMed] [Google Scholar]
- 19.Mego M, Cholujova D, Minarik G, et al. CXCR4-SDF-1 interaction potentially mediates trafficking of circulating tumor cells in primary breast cancer. BMC Cancer. 2016;16(1):127. doi: 10.1186/s12885-016-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 21.He L, Zhang E, Shi J, et al. (-)-Epigallocatechin-3-gallate inhibits human papillomavirus (HPV)-16 oncoprotein-induced angiogenesis in non-small cell lung cancer cells by targeting HIF-1α. Cancer Chemother Pharmacol. 2013;71(3):713–725. doi: 10.1007/s00280-012-2063-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Downregulation of NKAP hindered the impact of hypoxia on the MCF-7 cells.
Notes: (A) qRT-PCR demonstrating the mRNA expression of HIF-1, CXCR4, VEGF, and NKAP in normal (NC), hypoxic (Hypoxia), and hypoxic plus siNKAP transfected (Hypoxia + siNKAP) MCF-7 cells. (B and C) Western blot verified that siNKAP inhibited NKAP expression efficiently under hypoxia condition. (D) Cell proliferation detected by CCK8 assay. (E and F) Cell invasion detected by transwell assay. (G and H) Western blot image and quantification assay indicated the activation of AKT/mTOR signaling pathway. Magnification 100×. *P<0.05 compared with NC.